The Kinetic Molecular Theory of Matter
The theory states that matter is made up of tiny particles called molecules which are in constant motion.
Fundamental Assumptions of the Kinetic Molecular Theory
(i) Matter exists either in solid, liquid or gaseous state.
(ii) All substances consist of molecules, the smallest particle which can exist independently.
(iii) In solids the molecules vibrate about a mean or fixed position. The forces between the molecules are strong and may be attractive or repulsive. All true solids have a crystalline structure in which the atoms are arranged in regular patterns called lattices.
(iv) In liquids the molecules move freely in all directions. In addition to vibrational energy, they have translational energy. The Kinetic energy of the liquid molecules is greater than in solids.
(v) In gases the molecules are in constant motion and are further apart than in solids and liquids. They move at high speeds and have translational, vibrational and in addition rotational energy if the molecules are made of two or more atoms. The attractive or cohesive force is negligible, so, gases are perfectly free to expend and completely fill the vessels containing them. Gas molecules have the greatest Kinetic energy. Because the intermolecular forces are small, the motion of molecules in the gaseous state is linear until collision takes place either with other molecules or with the walls of the container.
Basic Assumptions of the Kinetic Theory of Gases
The Kinetic theory of matter has been more completely developed for gases than for solids and liquids. This is because the problems involved are much simpler in the case of gases. The simplest substance to which the theory has been applied is the ideal gas. The fundamental assumptions of the theory are as follows:
(i) Gases consist of many very small particles called molecules, which are like perfectly elastic spheres and are usually in constant random motion.
(ii) Molecules exert no forces on one another except when they collide. Therefore, between collisions with other molecules or with the walls of the container, they move in straight lines.
(iii) Collisions of molecules with one another or with the walls of the container are perfectly elastic. This means that the total Kinetic energy of two molecules before collision is the same as that after collision, and that when a molecule collides with the wall its Kinetic energy is unchanged.
(iv) The duration of a collision is negligible compared with the time between collisions
(v) Molecules are separated by distances which are very large compared with the size of the molecules (or the volume of the molecules is negligible when compared with the volume of the container); they are, however, distributed uniformly throughout the container.
(vi) Any finite volume of the gas contains a very large number of molecules. This assumption is supported by experimental evidence because under standard temperature and pressure (s.t.p), there are about 3 x 1019 molecules per cm3 of any gas.
Characteristics of the Three States of Matter
| S/N | Solids | Liquids | Gases | |
| 1. | Have definite shape | Have no definite shape. They take the shape of their container. | Have no definite shape | |
| 2. | Have fixed size and volume | Have fixed size and volume | Have no fixed size and volume but spread easily and occupy the volume of their container | |
| 3. | They don’t move easily | They can move easily | They move faster than liquids | |
| 4. | The molecules are closely packed and held together by strong intermolecular forces | Intermolecular distances are greater than that of solids but intermolecular forces are weaker than that of solids | Intermolecular distances are the farthest and intermolecular forces are weak and negligible | |
| 5. | They do not mix with other solids | They may mix or not mix with other liquids | Mix easily with other gases | |
| 6. | They are compressible | They are incompressible | They are compressible | |
Crystalline and Amorphous Substances
Solids are usually classified into two groups:
(i) Crystals or crystalline solids;
(ii) Noncrystal or non-crystalline solids;
The difference between crystals and non-crystals is the arrangement of atoms or molecules in the solid.
Crystals
Definition of Crystals
A crystal is a piece of solid matter in which the atoms, molecules or ions are arranged in a highly regular repeating pattern called lattice.
Crystal Lattice
The particles in a crystal are arranged in regular 3-dimensional framework or pattern called crystal lattice which repeats over and over again in all directions. The high degree of regularity and order in the arrangement of the molecules is the principal feature distinguishing solids from liquids. Particles in a liquid are jumbled and highly disorganized as they move about. They are even more disorganized in a gas. Examples of common crystals are: sodium chloride, zinc sulphide, chromium, iron and platinum salts.
Structure of Simple Crystals
A simple crystal is made up of a huge number of simple basic units or building blocks called unit cells. If you stack these units up and, down, side by side and in all directions, you can build the whole lattice. Unit cells are of three types, giving rise to 3 types of lattice and hence 3 types of crystals, simple cubic lattice, face-centered cubic lattice, and body-centered cubic lattice.
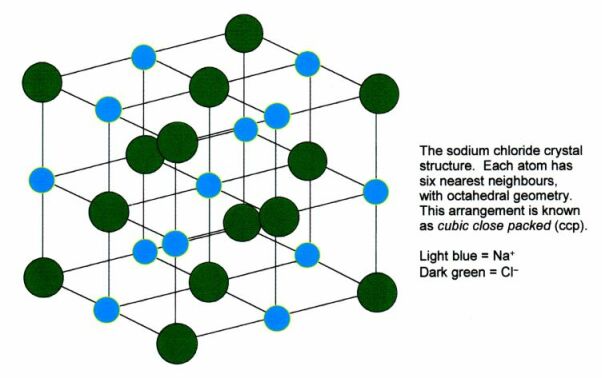
Sodium Chloride structure
a) Simple Cubic Crystal
Here the atoms or molecules or ions are placed at the corners of imaginary cubes stacked side by side, up and down like building blocks. An example is the Sodium chloride (NaCl) crystal. In the lattice, the atoms of Na and CI alternate positions in the cube in each of the 3 directions. Each atom within solid, thus has six immediate neighbors.
(b) Face-Centered Cubic Crystal
The unit cell has identical particles at each of the corners plus another particle in the center of each face as shown in the figure above. A typical face-centered cubic.
Crystal is Zinc sulphide (ZnS). Other crystals in this group include crystals of common metals like copper, silver, aluminum, lead, etc. Body-centered cubic crystal
Zinc Sulphide
(c) Body-centered Cubic Crystal
The unit cell has identical particles at each corner of the cube plus one in the center of the cell as illustrated in the figure include; chromium, iron and platinum salts.
Non-Crystalline and Amorphous Solids
The atoms of non-crystals are not regularly arranged as in the case of crystals. They are said to be “amorphous” that is having no definite shape or form; not organized. In a number of ways amorphous substances resemble liquids more than solids. Examples of amorphous solids are glass and plastics. Amorphous solids never form crystals.
They are usually made up of long, chain-like molecules that are intertwined in the liquid state just like strands of earthworms.
Note: Crystalline substances have high melting points because much heat is required to break the strong intermolecular forces binding the molecules together.
Differences between Amorphous and Crystalline Substances
| S/n | Crystalline Substances | Amorphous Substances | |
| 1 | They have definite shape | No definite shape | |
| 2 | They have definite and high melting points | They have no definite melting point | |
| 3 | They are usually soluble | They are not usually soluble | |
| 4 | They are either hydrated or anhydrous | All are anhydrous | |
| 5 | Crystallization takes place when melted | Crystallization never takes place when melted | |
EVALUATION
- Define molecule.
- State the kinetic molecular theory of matter.
- What are crystals?
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com