Carbohydrates (saccharides) – Molecules consist of carbon, hydrogen and oxygen atoms. It is a major food source and a key form of energy for most organisms. When combined together to form polymers, carbohydrates can function as long term food storage molecules, as protective membranes for organisms and cells, and as the main structural support for plants and constituents of many cells and their contents. Carbohydrates are naturally occurring organic compounds. The general molecular formular is Cx(H2O) y.
Types of Carbohydrates
Carbohydrates can be classified into two i.e. simple and complex sugars.
- Simple carbohydrates: These are also called simple sugars. They are crystalline, soluble in water and have a sweet taste. Structurally, they can be further divided into monosaccharides e.g. glucose, and disaccharides e.g. sucrose
- Complex carbohydrates: These are also called polysaccharides. They are non-crystalline, insoluble and tasteless substances, e.g. starch and cellulose. Starches include grain products, such as bread, crackers, pasta, and rice.
Monosaccharides
A monosaccharide or simple sugar has a formula that is some multiple of CH2O. For instance, glucose (the most common monosaccharide) has a formula of C6H12O6. This is the smallest possible sugar unit. Examples include glucose, galactose or fructose. Monosaccharides cannot be split into smaller units by the action of dilute acids. Monosaccharides are classified according to the number of carbon atoms they possess: trioses have three carbon atoms; tetroses, four; pentoses, five; hexoses, six; etc. Each of these is further divided into aldoses and ketoses, depending on whether the molecule contains an aldehyde group (–CHO) or a ketone group (–CO–). For example glucose, having six carbon atoms and an aldehyde group, is an aldohexose whereas fructose is a ketohexose. These aldehyde and ketone groups confer reducing properties on monosaccharides: they can be oxidized to yield sugar acids.

Glucose
Glucose commonly known as grape sugar or dextrose is present in fruits such as grapes, honey and also sap of plants. It is the main source of energy for mineral tissues and its present in the blood of animals.
Preparation
Glucose can be prepared from the hydrolysis of starch with dilute acid.
(C6H10O5)n + nH2O → nC6H12O6
Properties
If Glucose is heated with concentrated teteraoxosulphate(vi) acid, it will be dehydrated to form a black residue of carbon.
Disaccharides
A disaccharide is a derived or condensed from two molecules of monosaccharides by the elimination of one molecule of water.
monosaccharide + monosaccharide ↔ dissacharide + water
Sucrose
Sucrose or cane sugar is the common granulated sugar which we use to sweeten food. It occurs naturally in many plants and fruits. e.g. pineapple, carrots, sorghum and sap of sugar maple tree.
Preparation
Sucrose is prepared from juices of sugar cane and sugar beet. The cane or beet is shredded and crushed between rollers and the juice is extracted with water warmed to about 800C. The solution is then purified by treatment with slaked lime and carbon(iv) oxide. The purified solution is concentrated by distillation under reduced pressure. On cooling, the concentrated solution, brown crystals of sugar separate out. The remaining liquid called molasses still contains a reasonable amount of sugar and is used in ethanol production. The brown sugar obtained is impure. It is refined by treatment with slaked lime and carbon (iv) oxide, and decolorized with animal charcoal.
Properties
Sucrose is a colourless crystalline solid. It has a very sweet taste and dissolves readily in water but not alkanol.
Sucrose chars on strong heating or warming with concentrated tetraoxosulphate (vi) acid.
If sucrose is heated to a temperature of about 210 celcius which is above melting point but below its charring temperature, a yellowish – brown substance known as caramel. Caramel is used for flavouring and in confectionery.
sucrose + water ↔ glucose + fructose
Sucrose is used to sweeten food and beverages, and for preserving food. It is also used to produce ethanol by fermentation.
Polysaccharides
Preparation of Starch
The raw material to be used is peeled cassava tubers which should be washed and ground into pulp. Water is then added to the pulp to extract starch. It forms suspension and this can stay for sometime before the water above is decanted and starch residue is allowed to dry.
Physical Properties of Starch
- Starch is a white odourless, tasteless powder with the formula (C6H10O5)n
- It is insoluble in cold water but soluble in hot water forming a viscous solution which sets into a jelly on cooling
Chemical Properties of Starch
- Starch gives the familiar characteristics deep blue colour with iodine solution
- Hot dilute acids hydrolyse starch into maltose and glucose
- It does not reduce Fehling’s solution
- It decomposes on heating in the presence of the enzyme diastase to form maltose sugar.
Test for Starch
Add a few drops of iodine to some boiled starch, a dark blue colouration which disappears on cooling results.
Uses of Starch
- It is used for stiffening linen
- It is used to produce ethanol and glucose
- It is used mainly as food
Cellulose
Cellulose is the highest of the polysaccharides. It is the main component of plant cell walls and plant fibres. The principal industrial sources are cotton and wood each of which contains about 50% of cellulose. Other sources of cellulose for textile purposes are floxi china grass, hemp and jute.
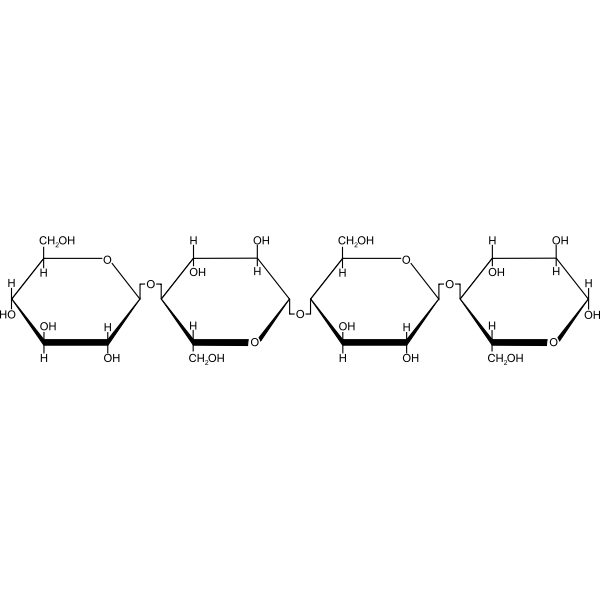
Physical Properties of Cellulose
- It forms transparent fibres when it is pure
- It is insoluble in water and in most organic solvents
Chemical Properties of Cellulose
- Cellulose can be completely hydrolysed to glucose by hot acids
- Hydrolysis of cellulose can also be carried out readily by the enzyme cellulose which is produced by micro organisms present in the digestive system of termites and herbivorous animals.
Uses of Cellulose
- It is used in the manufacture of explosives, surface coatings, paper, textiles and ropes
- In the manufacture of gum, cotton and explosives
ASSESSMENT (POST ANSWERS BELOW USING THE BOX)
- Chemical formular for starch is ………
a. (C5H10O5)n
b. (C6H12O5)n
c. (C6H10O5)n
d. (C6H11O5)n - A …………… is derived or condensed from two molecules of single sugar by the elimination of one molecule of water.
a. Disaccharide
b. Monosaccharide
c. Polysaccharide
d. Starch - Carbohydrates consists of ……
a. C, H, N
b. C, H, O
c. C and H
d. C and O - sucrose + water ↔ glucose + ………
a. glucose
b. galactose
c. fructose
d. maltose - Test for starch -Add a few drops of iodine to some boiled starch, a ……….. colouration which disappears on cooling results
a. Blue
b. Bluish-green
c. Dark green
d. Dark blue
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 