To account for the phenomena of electrolysis the Ionic Theory was put forward by Arrhenius in 1880. The theory states that electrolytes are made up of ions, which are built up in certain patterns called crystal lattice. When these substances dissolve in water, the structure is destroyed and the ions are set free to move.
Concentrated mineral acids such as sulphuric acid, hydrochloric acid and nitric acid do not contain ions but they consist of molecules. However, when they are diluted, the molecular structure is destroyed and ions are formed.
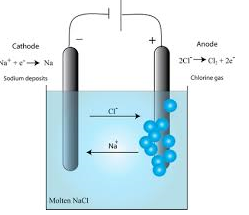
THE MECHANISM OF ELECTROLYSIS
The conductivity of ionic compounds is explained by the fact that ions move in a particular direction in an electric field. This can be shown in experiments with coloured salts. For example, copper (II) chromate (VI) (CuCrO4) dissolves in water to give a green solution. This solution is placed in a U-tube. A colourless solution of dilute hydrochloric acid (HCl) is then layered on top of the salt solution in each arm. Graphite rods are fitted as shown in figure 13.3. These rods (electrodes) carry the current into and out of the solution.
After passing the current for a short time, the solution around the cathode becomes blue. Around the anode, the solution becomes yellow. These colours are produced by the movement (migration) of the ions in the salt. The positive copper ions (Cu2+) are blue in solution. They are attracted to the cathode (negative electrode). The negative chromate ions (CrO42-) are yellow in solution. They are attracted to the anode (the positive electrode). The use of coloured ions in solution has shown the direction that positive and negative ions move in an electric field. Always positive ions (cations) move to the cathode and negative ions (anions) move to the anode.

Figure 6.3: The movement of ions in solution
Electrovalent compounds
- Electrovalent compounds are formed by complete transfer of electrons in KCI (K. + .CI: — K+ CI–)
- Electrovalent compounds are made up of ions.
- Electrovalent compounds are heard, crystalline solids e.g. NaCI, MgCl2
- Electrovalent compounds are usually soluble in water but insoluble in non-polar solvents like CI4
- Electrovalent compounds generally have high melting and boiling points.
- Electrovalent compounds are good conductors of electricity in the molten state and in aqueous solutions but insulators in the solid state.
Covalent compounds
- Covalent compounds are formed by mutual sharing of electrons as in NH3
- Covalent compounds are made up of molecules.
- Covalent compounds are usually liquids or gases. E.g. CH4, C2 H6, NH3
- Covalent compounds are soluble in non-polar solvents like benzene or carbon tetrachloride and in soluble in polar solvents like water.
- Covalent compounds generally have low melting and boiling points.
- Covalent compounds are bad conductors of electricity.
Electrovalent Compound
The compounds which contain ionic or electrovalent bonds are known as Electrovalent or Ionic Compounds. Mainly electrovalent compounds are formed due to reaction between highly electropositive and highly electronegative atoms.
Characteristics of Electrovalent Compounds
1. Crystal Structure:
In solid state of electrovalent compounds anions and cations are arranged in regular manner called as crystal, In which anions surrounded by definite number of cations and cations surrounded by definite number of anions.
2. Physical Nature:
Ionic or electrovalent compounds are generally hard and their hardness increases with increasing ionic charge and decreasing distance between ions.
3. Solubility:
Positive ion of ionic compound attach with negative part of polar solvent and negative ion of ionic compound attach with positive part of polar solvent, so ionic or electrovalent compounds are soluble in polar solvents like water and insoluble in non polar solvents like benzene, ether, alcohol.
4. Melting Point and Boiling Point:
Electrovalent or ionic compounds have high Melting and boiling points because they need large amount of energy to break strong ionic bonds.
5. Electrical Conductivity:
In molten and solution forms electrovalent compounds conduct electricity because ions flows in molten and solution forms.
Characteristics of Covalent Compounds
1. Crystal Structure:
Crystal structure of covalent compounds is formed from atoms or molecules. Crystal of covalent compounds are divided in three parts as –
- These are crystals of covalent compounds whose molecule are very small and these molecules are held together by vander waals forces.
Example: Sulphur, Iodine.
- These are crystals of covalent compounds whose molecule are very large due to combination of every atom with other atom by covalent bonds.
Example: Diamond, Silica.
iii. These are crystals of covalent compounds whose have separate layers.
Example: Graphite.
2. Physical Nature:
Due to weaker force of attraction between the molecules of the covalent compounds, maximum covalent compounds are gases or liquids but some covalent compounds exist as solid like Urea, Sugar, Glucose, and Naphthalene.
3. Solubility:
Covalent compounds are not soluble in polar solvents like water but are soluble in non-polar solvent like alcohol, ether, carbon tetra chloride.
4. Melting Point and Boiling Point ( MP and BP) :
Melting and boiling points of covalent compounds are very low because very less energy is required to overcome the weak force of attraction between the neutral molecules in the covalent compound. But Diamond and Graphite are exception because they have very high melting and boiling points.
5. Conductivity:
Covalent compounds do not have ions so they do not conduct electricity but some polar covalent compounds conduct very less electricity.
Strong Electrolytes
Strong electrolytes include the strong acids, strong bases, and salts. These chemical completely dissociate into ions in aqueous solution.
Examples
- hydrochloric acid – HCl
- hydrobromic acid – HBr
- hydroiodic acid – HI
- sodium hydroxide – NaOH
- strontium hydroxide – Sr(OH)2
- sodium chloride – NaCl
Weak Electrolytes
Weak electrolytes only partially break into ions in water. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Most compounds that contain nitrogen are weak electrolytes.
Examples
- HF – hydrofluoric acid
- CH3CO2H – acetic acid
- NH3 – ammonia
- H2O – water (weakly dissociates in itself)
Glucose is a nonelectrolyte.
Nonelectrolytes
Nonelectrolytes do not break into ions in water. Common examples include most carbon compounds, such as sugars, fats, and alcohols.
Examples
- CH3OH – methyl alcohol
- C2H5OH – ethyl alcohol
- C6H12O6 – glucose
Conductors and non-conductors (insulators)
We have seen in the above definitions that there are some substances that can conduct electricity while others cannot. For a solid to conduct electricity, it must have a structure that contains “free” electrons that are able to flow through it. All metals conduct electricity whether in solid or molten states. They are electrical conductors.
The structure of a solid metal has positive ions surrounded by a ’sea’ of electrons.
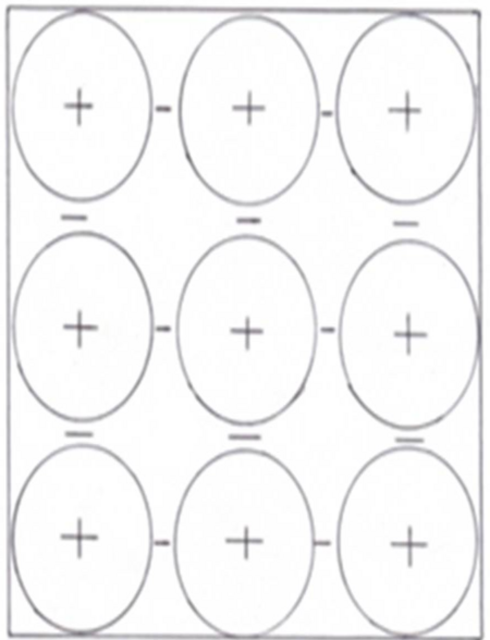
Figure 6.1: Positive ions surrounded by free electrons
The electrons between the positive ions are free to move. When a metal is connected to the + and – of a power supply a current flows. The electrons flow through the metal.
Nearly all non-metals do not conduct electricity. They are electrical insulators. They do not have freely moving electrons to carry the current.
Graphite is the only no-metal that conducts electricity. This solid has unusual structure. The atoms of graphite are joined in layers by covalent bonds, but between the layers, there are weak bonds with electrons that can move freely, allowing electrical current to flow.
When electricity flows through a solid or liquid metal or through solid graphite no chemical reactions takes place. The copper wire is still copper when the current is switched off.
Electrolytes and non-electrolytes
Liquids such as ethanol, paraffin, petrol and methylbenzene do not conduct electricity. The bonding in these compounds is covalent. These substances consist of molecules. There are no free electrons or charged particles to flow through them. Solutions of covalent compounds, for example sugar solution, do not conduct electricity. These compounds are non-electrolytes. Non-electolytes exist only in the form of molecules and are incapable of ionization. Examples of no-electrolytes include trichloromethane, CH3Cl, cane sugar, (C12H22O11), alcohol (ethanol), (C2H5OH) and urea (carbamide) (CON2H4)
Ionic compounds contain charged particles (ions), but in solid state, the ions are firmly held in place and they are not free to move. An ionic solid does not conduct electricity. However, the ions present can become free to move if the solid is melted or dissolved in water. Then they can conduct electricity. For example, solid sodium chloride cannot conduct electricity but when melted or dissolved in water, the ions, Na+ and Cl– are set free. Then these ions are free to move in solution and hence conduct electricity. These compounds are called electrolytes.
Weak and strong electrolytes
Weak electrolytes are compounds that are only partially or slightly ionized in aqueous solutions. Some substances, for example, ethanoic acid solution ionize partially.
CH3COOH(aq) ⇔ CH3COO–(aq) + H+(aq)
Most of the electrolytes exist in solution in the form of unionized molecules. For example, in ordinary dilute (2M) ethanoic acid, out of every 1000 molecules present, only 4 are ionized and 996 are unionized.
A solution of ammonia water is also a weak electrolyte, containing a relatively small proportion of ammonium and hydroxyl ions.
NH4OH(aq) ⇔ NH4+(aq) + OH–(aq)
Most of the organic acids are weak electrolytes, e.g. tartaric, citric and carbonic acids.
However, there is no sharp dividing line between weak and strong electrolytes.
Water is also a weak electrolyte. It ionizes only slightly.
H2O(l)⇔ H+(aq) + OH–(aq)
Study shows that for every molecule of water ionized, there are 6 million molecules of water not ionized.
Strong electrolytes are compounds that are completely ionized in aqueous solutions. When sodium chloride is dissolved in adequate water it ionizes completely into Na+ and Cl– ions. There are no NaCl solid particles left unionized. All strong electrolytes (salts, the mineral acids and caustic alkalis) ionize completely in solutions.
EVALUATION
Define the following terms
i.electrolytes ii. non-electrolytes iii. weak electrolytes iv. conductors v. covalent compounds vi .electrovalent compounds
Differenciate between an electrolyte and a conductor
Describe the principle behind the ionic theory.
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 