In our daily life we come across processes like rusting of iron articles, fading of the colour of the clothes, burning of the combustible substances such as cooking gas, wood, coal, etc. All these processes fall in the category of redox reactions. A large number’ of industrial processes like, electroplating, extraction of metals like aluminium and sodium, bleaching of wood pulp, manufacture of caustic soda, etc., are also based upon the redox reactions: Redox reactions also form the basis of electrochemical and electrolytic cells. In the present unit we shall understand the meaning of oxidation, reduction, oxidising agents and reducing agents.
CLASSICAL CONCEPT OF OXIDATION AND REDUCTION
According to classical concept following definitions were proposed to explain the process of oxidation and reduction.
Oxidation: It is a process of chemical addition of oxygen or any electronegative radical or removal of hydrogen or any electropositive radical.
Reduction: It is a process of chemical addition of hydrogen or any electropositive radical or removal of oxygen or any electronegative radical.
Some examples of oxidation and reduction reactions are given below:
(i) Reaction of PbO and carbon
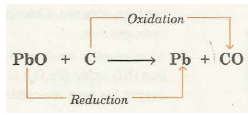
Here, oxygen is being removed from lead oxide (PbO) and is being added to carbon (C). Thus, PbO is reduced while C is oxidised.
(ii) Reaction of H2S and Cl2
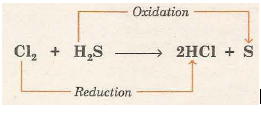
Here, hydrogen is being removed from hydrogen sulphide (H2S) and is being added to chlorine (C2S). Thus, H2S is oxidised and Cl2 is reduced.
(iii) Reaction between Mg and F2
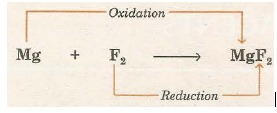
Here, electronegative radicals fluoride ion (p) is added to magnesium while electropositive radical Mg2+ is added to fluorine. Hence, Mg is oxidised and F2 is reduced.
Looking at all the reactions given above we observe that oxidation and reduction occurs simultaneously, hence the word “redox” was coined for this class of chemical reactions.
OXIDATION-REDUCTION IN TERMS OF ELECTRON TRANSFER
In order to understand electronic concept of oxidation and reduction let us study the reaction of magnesium with oxygen. When magnesium is burnt in oxygen it gets oxidised to magnesium oxide (MgO). In the formation of magnesium oxide, two electrons from magnesium atom are transferred to oxygen atom
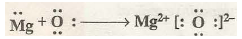
The process of transference of electrons is described as redox process. Let us define oxidation and reduction in terms of electrons.
Oxidation is a process in which an atom or a group of atoms taking part in chemical reaction loses one or more electrons. The loss of electrons results in the increase of positive charge or decrease of negative charge of the species.
For example,
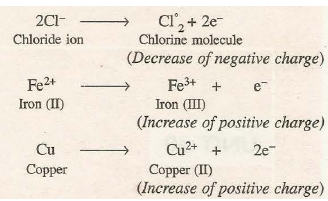
The species which undergo the loss of electrons during the reactions are called reducing agents or reductants. Cl-, Fe2+ and Cu are reducing agents in the above examples.
Reduction is a process in which an atom or a group of atoms taking part in chemical reaction gains ·one or more electrons. The gain of electrons results in the decrease of positive charge or increase of negative charge of the species. For example,
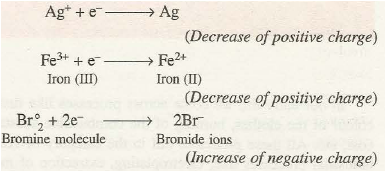
The species which undergo gain of electrons during the reactions are called oxidising agents or oxidants. In the above examples, Ag+, Fe3+ ions, Br2 molecule are oxidising agents.
SIMULTANEOUS OCCURRENCE OF OXIDATION AND REDUCTION
Since oxidation involves loss of electrons and reduction involves gain of electrons, it is evident that if one substance loses electrons, another substance at the same time must gain electrons because electrons cannot be the products in any chemical change. This means that in any process, oxidation can occur only if reduction is also taking place side by side and vice versa. Thus, neither oxidation, nor reduction can occur alone. Both the processes are complementary like give and take and proceed simultaneously. That is why chemical reactions involving reduction-oxidation are called redox reactions. In fact, during the redox reaction there is a transference of electrons from the reducing agent to the oxidising agent as shown below:
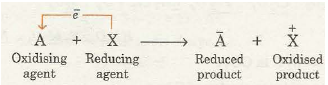
For example, consider a reaction between zinc and copper ions
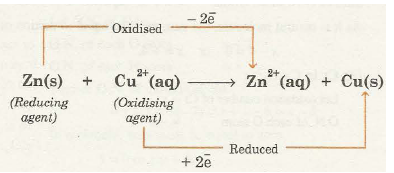
In this reaction zinc atoms lose electrons and are oxidised to zinc ions (Zn2+) whereas cupric ions (Cu2+) gain electrons and are reduced to copper atoms. Thus, cupric ions act as oxidising agent and zinc atoms act as reducing agent. In fact the oxidising agent gets reduced while reducing agent gets oxidised during the redox reactions.
Oxidation Number or Oxidation State
In many covalent reactions such as reaction between H2 and Cl2:
H2(g) + Cl2(g) à 2HCl(g)
the loss and gain of electrons could not be easily explained. In order to explain transference of electrons in either of the species in a more convenient way, the concept of oxidation number has been introduced.
Oxidation number (O.N.) of the element is defined as the residual charge which its atom has or appears to June when all other atoms from the molecule are assumed to be removed as ions by counting the shared electrons with more electronegative atom.
For example, in hydrogen chloride molecule, chlorine is more electronegative than hydrogen. Therefore, the shared pair is counted towards chlorine atom as shown below:
As a result of this, chlorine gets one extra electron and acquires a unit negative charge. Hence, oxidation number of chlorine is -1. On the other hand, hydrogen atom without electron has a unit positive charge. Hence, oxidation number of hydrogen in hydrogen chloride is + 1.
It may be noted that electrons shared between two similar atoms are divided equally between the sharing atoms. Hence in molecules like H2, Cl2, Br2 the oxidation number of element is zero.
RULES FOR ASSIGNING OXIDATION NUMBER TO AN ATOM
These rules have been formulated on the basis of the assumption that electrons in a covalent bond belong entirely to the more electronegative atom.
- The oxidation number of the element in the free or elementary state is always zero irrespective of its allotropic form.
For example,
Oxidation number of helium in He = 0
Oxidation number of chlorine in Cl2 = 0
Oxidation number of sulphur in S8 = 0
Oxidation number of phosphorus in P4 = 0
- The oxidation number of the element in monoatomic ion is equal to the charge on the ion. For example, in K+CI-, the oxidation number of K is + 1 while that of Cl is -1. In the similar way, oxidation number of all the alkali metals is + 1 while those of alkaline earth metals is +2 in their compounds.
- The oxidation number of fluorine is always -1 in all its compounds. Other halogens (Cl, Brand I) also have an oxidation number of -1, when they occur as halide ions in their compounds. However, in oxoacids and oxoanions they have positive oxidation numbers.
- Hydrogen is assigned oxidation number + 1 in all its compounds except in metal hydrides. In metal hydrides like NaH, MgH2 , CaH2, LiH, etc., the oxidation number of hydrogen is -1.
- Oxygen is assigned oxidation number -2 in most of its compounds, however, in peroxides (which contain 0-0 linkage) like H2O 2, BaO2, Na2O2, etc., its oxidation number is -1. Similarly, the exception also occurs in compounds of fluorine and oxygen like OF2 (F-0-F) and 0 2F2 (F-0- 0 – F) in which the oxidation number of oxygen is +2 and+ 1 respectively.
- In accordance with principle of conservation of charge, the algebraic sum of the oxidation numbers of all the atoms in molecule is zero. But in case of polyatomic ion the sum of oxidation numbers of all its atoms is equal to the charge on the ion.
- In binary compounds of metal and non-metal, the metal atom has positive oxidation number while the non-metal atom has negative oxidation number. For example, O.N. of Kin KI is +1 but O.N. of l is – l.
- In binary compounds of non-metals, the more electronegative atom has negative oxidation number, but less electronegative atom has positive oxidation number. For example, O.N. of Cl in CIF3 is positive (+3) while that in ICl is negative (-1).
By the application of above .rules, we can find the oxidation number of the desired element in a molecule or in an ion. It may be noted that if a molecule contains two or more atoms of same element, then oxidation number calculated by these rules is average oxidation number.
Let us apply the above rules to calculate the oxidation number of some elements.
Oxidation and Reduction in Terms of Oxidation Number
After having discussed the concept of oxidation number, let us now define oxidation and reduction in terms of oxidation number. Oxidation is defined as a chemical process in which oxidation number of the element increases. On the other hand, reduction is defined as the chemical process in which oxidation number of the element decreases. For example, let us consider the reaction between hydrogen sulphide and bromine to give hydrogen bromide and sulphur.
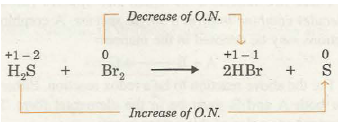
In the above example, the oxidation number of bromine decreases from 0 to -1, thus, it is reduced. The oxidation number of S increases from – 2 to 0. Hence, ~S is oxidised.
Let us now define oxidising and reducing agents in the light of the concept of oxidation number:
Oxidising agent is a substance which undergoes decrease in the oxidation number of one or more of its elements.
Reducing agent is a substance which undergoes increase in the oxidation number of one or more of its elements. In the above example, H2S is reducing agent while Br2 is oxidising agent.
OXIDATION HALF AND REDUCTION HALF REACTIONS
Every redox reaction can be split up into two half reactions. The part of the redox reaction which represents loss of electrons or increase in oxidation number, is called oxidation half reaction while the other part which represents gain of electrons or decrease in oxidation number, is called reduction half reaction. Some examples are given below:
(i) The reaction: Zn + Cu2+ àZn2+ + Cu, can be split up into two half equations as

(i) The reaction: Sn2+ + 2Hg2+ à Sn4+ + Hg2 can be split up into half reactions as
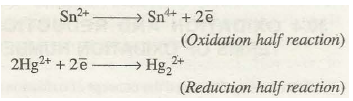
OXIDATION NUMBER AND IUPAC NOMENCLATURE
“Oxidation state” is defined as the charge an atom might be imagined to have when electrons are counted according to an agreed-upon set of rules:
- the oxidation state of a free element (uncombined element) is zero
- for a simple (monatomic) ion, the oxidation state is equal to the net charge on the ion
- hydrogen has an oxidation state of +1 and oxygen has an oxidation state of −2 when they are present in most compounds. Exceptions to this are that hydrogen has an oxidation state of −1 in hydrides of active metals, e.g. LiH, and oxygen has an oxidation state of −1 in peroxides, e.g. H
2O
2. - the algebraic sum of oxidation states of all atoms in a neutral molecule must be zero, while in ions the algebraic sum of the oxidation states of the constituent atoms must be equal to the charge on the ion.
Determining the oxidation state or number
There are two different methods for determining the oxidation state of elements in chemical compounds. First (and widely taught) a method based on the rules in the IUPAC definition (see above). Second, a method based on the relative electronegativity of the elements in the compound, where the more electronegative element is assumed to take the negative charge.
Simple examples using IUPAC definition
- Any pure element—even if it forms diatomic molecules like chlorine (Cl
2)—has an oxidation state of zero. Examples of this are Cu or O
2. - For monatomic ions, the oxidation state is the same as the charge of the ion. For example, the sulfide anion (S2−
) has an oxidation state of −2, whereas the lithium cation (Li+
) has an oxidation state of +1. - The sum of oxidation states for all atoms in a molecule or polyatomic ion is equal to the charge of the molecule or ion. Thus, the oxidation state of one element can be calculated from the oxidation states of the other elements.
- An application of this rule is that the sum of the oxidation states of all atoms in a neutral molecule must be zero. Consider a neutral molecule of carbon dioxide, CO
2. Oxygen is assumed to have its usual oxidation state of −2, and so the sum of the oxidation states of all the atoms can be expressed as x + 2(−2) = 0, or x − 4 = 0, where x is the unknown oxidation state of carbon. Thus, it can be seen that the oxidation state of carbon in the molecule is +4. - In polyatomic ions, the sum of the oxidation states of the constituent atoms must be equal to the charge on the ion. As an example, consider the sulfate anion, which has the formula SO2−
4. As indicated by the formula, the total charge of this ion is −2. Because all four oxygen atoms are assumed to have their usual oxidation state of −2, and the sum of the oxidation states of all the atoms is equal to the charge of the ion, the sum of the oxidation states can be represented as y + 4(−2) = −2, or y − 8 = −2, where y is the unknown oxidation state of sulfur. Thus, it can be computed that y = +6.
These facts, combined with some elements almost always having certain oxidation states (due to their very high electropositivity or electronegativity), allows one to compute the oxidation states for the remaining atoms (such as transition metals) in simple compounds.
Example for a complex salt: In Cr(OH)
3, oxygen has an oxidation state of −2 (no fluorine or O−O bonds present), and hydrogen has a state of +1 (bonded to oxygen). So, each of the three hydroxide groups has an overall oxidation state of −2 + 1 = −1. As the compound is neutral, chromium has an oxidation state of +3.
EVALUATION
- What is the value of X in the following equation?
2H2S + SO2→ 2H2O + XS
- 6 B. 4 C.3 D. 2
2.The oxidation number of phosphorus in H4P2O7 is
- +1 b.+2c. +5 d.+3 e.+4
3.Oxidation is a process involving
- gain of electrons b. loss of electrons c. gain of hydrogen d .loss of oxygen e.ion exchange
4.Define the terms oxidation and reduction
5.In the reaction Fe2O3(S)+3CO(g)-2Fe(s)+3CO2(g)
Which of the substances is
i.reduced and
ii .oxidized?
6.Name this compound H4P2O7
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 