KINETIC THEORY OF GASES
The kinetic theory of matter postulates that the tiny Particles of matter are continually moving and so possess kinetic energy. An increase in temperature causes an increase in the average kinetic energy of the particle.
Dalton’s experimental evidence shows that chemical compounds consists of molecules, which are groups of atoms of various elements. The gas laws which explained the physical behaviour of gases can be explained by kinetic theory of gases. This theory describes the behaviour of an ideal or perfect gas. This is to say that the kinetic theory of gases explains quantitatively the properties of gas molecules and in so doing put up the following assumptions.
EVALUATION
THE APPLICATION OF KINETIC THEORY
The kinetic theory is useful in several respects and such aspects includes;
PV=13NMC2
EVALUATION
PHENOMENA TO ILLUSTRATE THE KINETIC THEORY OF MATTER
The following phenomena illustrate the kinetic theory of matter
EVALUATION
BOYLE’S LAW
The relationship between volume and pressure of a gas was first started by Robert Boyle in 1662.
Boyle’s law states that the volume of a given mass of gas is inversely proportional to its pressure, provided that the temperature remains constant.
According to Boyle’s law, volume of a gas increases as the pressure decreases and vice versa.
This relationship is independent of the nature of the gas and it can be expressed mathematically as:
V∝1P∴V=kp
or PV=k
Where V = Volume at pressure P
K = A mathematical constant
For a given mass of a gas, the product of its pressure and its volume is always a constant. If the pressure of a given mass of gas increases, its volume will decrease by a similar proportion and vice versa, as long as the temperature remain constant. This relationship can also be expressed mathematically as:
P1V1=P2V2
Where V1 = Volume at pressure P1
V2 = Volume at pressure P2
Boyle’s law can still be re-stated as: ‘The pressure of a given mass of gas is inversely proportional to its volume, provided the temperature remains constant. Boyle’s law can further be illustrated with the diagram below, showing that when P is increasing, V is decreasing and when P is decreasing, V is increasing.
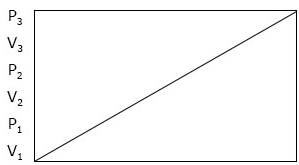
P1 = Initial pressure
P2 = Final pressure
V1 =Initial volume
V2 = final volume
Graphical representation of Boyle’s law

Graphical Representation of Boyles Law
Worked examples
P1V1=P2V2 (Boyle’s law)
P1 = 770mmHg
P2 = 750mmHg
V1= 375cm3
V2 = ? (new volume of gas)
P1V1=P2V2V2=P1V1P2=770×375750=385cm3
The new volume will be 385cm3
100cm3 of a gas has pressure of 1 atmosphere. Determine the volume of the gas at 5 atmospheres keeping the temperature constant.
Solution: since T is constant, we are to use Boyle’s law.
P1 Initial pressure = l atmosphere
P1 Final pressure = 5
V1 Initial volume = 100cm3
V2 (required quantity)
Recall: V2=P1V1=P2V2
V2=P1V1P2=100×15=20cm3
EVALUATION:
CHARLES’ LAW
The effect of temperature changes on the volume of a given mass of a gas at a constant pressure is described by Charles. Charles’ law states that the volume of a given mass of gas is directly proportional to its temperature in Kelvin, provided that pressure remains constant.
The volume of the gas decreases as the temperature decreases, and increases as the temperature increases.
Mathematically, the law can be expressed as:
V∝T∴V=kT
or VT=k
Where V = volume
T = Kelvin Temperature
K = mathematical constant
A Representation of Charles’s law
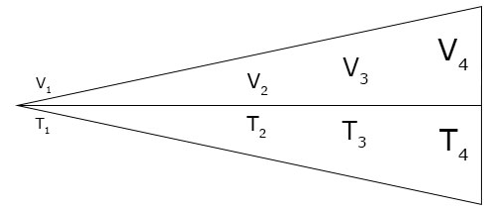
For a direct relationship, when the temperature increases, the volume will also increase at the same rate and vice versa, at constant pressure .The diagram above shows that when V is decreasing, T is also decreasing and when V is increasing, T is also increasing thus, making the quotient constant.
Charles’s law can be represented graphically has shown below.

If we divide the varying gas volumes by the corresponding temperature in Kelvin, the result would always be a constant. This relationship can also be expressed in another form.
V1T1=V2T2∴V2=T2V1T1
Where V1 is the volume at temperature T1
V2 is the volume at temperature T2
ABSOLUTE ZERO
This is the temperature at which the volume of a gas is theoretically zero..At this temperature there is no motiom of any form and all gases have been liquefied or solidified. The value of the temperature is -2730C.
TEMPERATURE CONVERSION
Where T = Temperature in Kevin
0C = Temperature in Celsius.
Examples:
(a) 1000C (b) 00C (c) -570C
Solution
Recall: T = 0C + 273
(a) 1000C = (100 + 273) = 373k
0C =(0 + 273) = (0 + 273) = 373k
(b) −570c = (−57 + 273)k = (273 − 57) = 216k
(a) 298k (b) 405k (b) 285k (d) 0k
Solution
Recall 00c = k – 273
298k = (298 – 273)0C = 250C
405k = (405 – 273)0C = 120C
0k = (0 – 273)0C = − 2730C
Worked examples on Charles’s law
Here pressure is constant. Charles’s law will apply.
V1 = 20.0dm3
T1 = 273k
T2 = 746
Recall Charles’s law V1T1=V2T2V2=T2V1T1V2=20×746273=40.0dm3
EVALUATION:
post your answers to the forum for review
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 