COMPOUND
A compound is a substance which contains two or more elements chemically combined together. A compound is formed as a result of a chemical change. It is a new substance with entirely different properties from those of substances from which it is formed. For example water is a compound of hydrogen and oxygen chemically combined in the ratio 2:1 by mass respectively. Other examples of compound are sound, limestone, common salt, petrol, kerosene, etc.
MIXTURE
A mixture is made up of two or more substances which can be mixed together, mechanically, in any proportion. It can be said to contain two or more constituents which easily be separated by physical method. Examples are air, soil, well water, tap water, milk, sweat, blood etc.
DIFFERENCES BETWEEN COMPOUNDS AND MIXTURES
| S/N | COMPOUNDS | MIXTURES | |
| 1 | Constituents are present in a fixed proportion by mass | Constituents can be mixed in any proportion | |
| 2 | Constituents are joined by chemical bonds | No chemical bond between constituents | |
| 3 | It is always homogeneous | It may be homogeneous or heterogeneous | |
| 4 | The properties differ entirely from those of its components elements | The properties are the sum of those of its individual constituents | |
| 5 | Constituents of compounds cannot be separated by physical means | Components of mixtures can be separated by physical means | |
EVALUATION:
SEPARATING A MIXTURE OF TWO SOLIDS
The following methods are employed in the separation of a mixture of two solids:
Purification by sublimation

Purification by sublimation
EVALUATION
SEPARATING A MIXTURE OF AN INSOLUBLE SOLID AND A LIQUID.
The Centrifugation Process

The Filtration Process
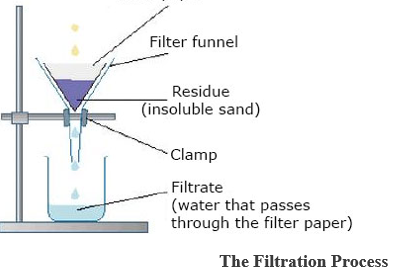
The Filtration Process
EVALUATION:
SEPARATING A SOLUBLE SOLID FROM A LIQUID
Evaporation is based on the large different between the boiling points of the solid and solvent. For example common salt can be recovered from its aqueous solution by complete evaporation of water. The solvent (liquid) is usually sacrificed

The Evaporation Process
Note: Evaporation method is not suitable for salts that can easily be destroyed by heating.
Note: If all the liquid is evaporated a powder will be obtained and not crystals. This powder might also contain impurities which otherwise would have remained in the solution and not contaminate the crystals. Many crystals formed on cooling saturated solution contain water which is chemically combined and loosely bonded to the crystals. This water is called water of crystallization. Salts which contain water of crystallization are said to be hydrated. Those which do not are anhydrous. Those are often powders.
EVALUATION:
SEPARATING A SOLUBLE SOLID FROM A LIQUID:
This is a method used to separate a mixture containing different soluble solid solutes in a liquid. The solubility of the different solid solutes in the given solvent must differ at different temperatures. The process of separation is the same as in crystallization process. While cooling the solution crystals of the relevant solid solutes will come out of the solution leaving behind the others which are still within their limits of solubility.
In physical precipitation, two solids that are soluble in the same solvent are separated by the addition of another solvent in which one of the solids is insoluble, e.g. an aqueous solution of common salts (sodium chloride) and green vitriol( a compound of iron). It is a method used to separate a solid which has a difference in solubility in two different miscible liquids. For example, ethanol and water are two miscible liquids. Iron(II) tetraoxosulphate(VI) is soluble in water but not in ethanol. On addition of ethanol to a solution containing a mixture of iron(II) tetraoxosulphate and water, the iron(II) tetraoxosulphate(VI) will be precipitated out and can be separated by filtration.
SEPARATING A MIXTURE OF TWO OR MORE LIQUIDS
Distillation is the evaporation of water or other liquids from a solution and its recovery on a pure state by condensation. The method of distillation is used to recover a solvent (liquid from a solution mixture). That is, a pure liquid from an impure liquid (mixture). The apparatus used are shown in the diagram below.
(a) Simple distillation process
A mixture of two liquids with widely differing boiling points can be separated by evaporating one from the other and re-condensing it in a separate vessel. The process is called simple distillation.
Distillation is carried out by condensing the vapour, using a condenser. The vapour which is condensed and collected in a separate vessel is called the distillate.
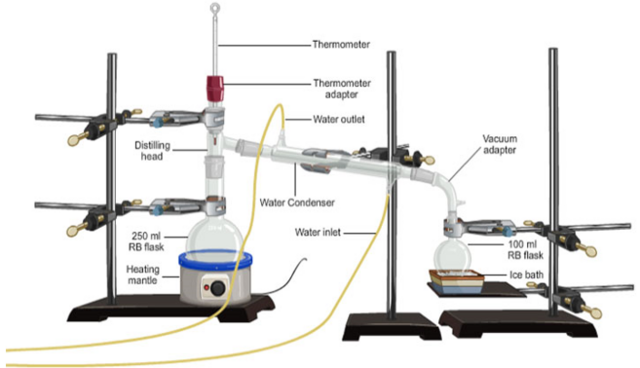
At the end of the distillation process the liquid that is collected at the end of the Liebig condenser is called the distillate. The solutes and other impurities are left behind in the distillation flask.
Differences between evaporation and distillation
| S/N | DISTILLATION | EVAPORATION | |
| 1 | Mainly for obtaining the solvent. | Mainly for obtaining salt from solution. | |
| 2 | It involves boiling and condensation. | It involves boiling only. | |
(b) Fractional Distillation
Fractional Distillation is a process used to separate a mixture of miscible liquids by a repeated evaporation and condensation making use of fractionating column (as shown in the diagram below)
Mixture of two or more miscible liquids are separated into its component parts. The liquids distill according to their boiling points starting with the liquid with the lowest boiling point. The apparatus used is the same as in distillation except for the presence of a fractionating column between the flask and the condenser.
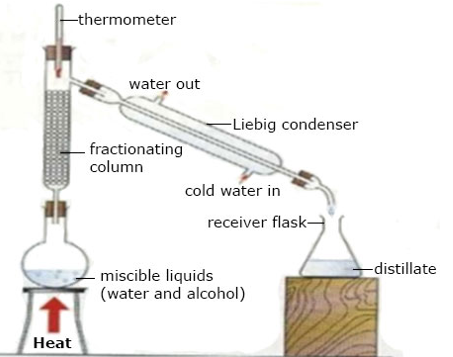
Fractional Distillation
Note: For efficient fractional distillation, the difference in the boiling points between successive fractions must be more than 100c.
EVALUATION:
SEPARATING IMMISCIBLE LIQUIDS (USING SEPARATING FUNNEL METHOD)
This a method used to separate a mixture of immiscible liquids e.g. a mixture of petrol and water. When the two liquids are added together they do not mix, instead they separate into two distinct layers, a lower denser layer and an upper less dense layer in the funnel as below.

Separating Immiscible Liquids (Separating Funnel Method)
EVALUATION:
Draw a labeled diagram to show how you would separate a mixture of kerosene and water.
CHROMATOGRAPHY
Separating complex mixtures by chromatography: This is a method of separation of the components of mixtures of solutes from a solution (mixture) using a solvent (liquid) moving over a porous, adsorbent medium e.g. filter paper or gel. This method can be mixtures of soluble substances. There are different types of chromatographic methods. Paper chromatography (ascending paper chromatography), column chromatography, thin layer chromatography and gas chromatography.
ASCENDING PAPER CHROMATOGRAPHY
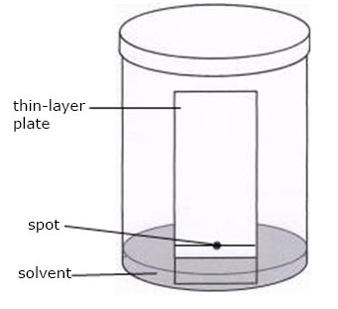
Ascending Paper Chromatography
As shown in the above diagram, the apparatus include: a glass jar with lid, filter paper, clips, solvent (water or ethanol). The solution containing the mixture of solutes to be separated is spotted unto the strips of paper near one end.
The paper is then suspended in a closed air- tight jar with the spotted end (but not the spot) dipping into the solvent. As the solvent ascends the paper the different solutes in the mixture gets dissolved and also more along the paper strip at different speeds and hence become separated. The paper strip is removed from the jar when the solvent has moved about three-quarters way up the strip. It is dried and if necessary sprayed with appropriate chemical reagents to locate the positions of the various components along the strip. Each solute can then be identified by the distance it has traveled. This is done by comparing its distance with those of known standard substances.
EVALUATION
1. Name the most suitable physical method for each of the following. (a) Containing groundnut oil from a mixture of the oil and water. (b) Obtaining pure water from sea water.
2. Draw the laboratory set up most suitable for each of the following. (a) Separating of a mixture of palm oil and water (b) Separate of pure liquid from an impure liquid.
3. State one industrial application of each of the following methods of separation explaining clearly the procedure. (a) crystallization (b) filtration (c) fractional distillation (d) evaporation
4. With the aid of a labelled diagram only show how pure sample of ethanol (alcohol) can be obtained from a mixture of ethanol and water.
5. Why is sodium chloride solution regarded as a mixture? (b) Draw a labelled diagram to show how pure sodium chloride can be obtained from its solution.
FLOATATION
Floatation method is based on the wide difference in the densities of the components of the mixture. The method is used for the separation of a mixture of two solids in which one component is light and the other is heavy. On the addition of a liquid in which neither is soluble, one component sinks, while the other floats. e.g. a mixture of coarse sand and wooden cork.

PROCEDURE: Place the mixture in a beaker and add plenty of water. The sand particles sink, while the wooden corks float.
FROTH FLOTATION (FROSTATION)
This method is specifically used to separate an ore of a metal from earthy impurities.
PROCEDURE: The ore is crushed into powder and then mixed with water containing detergent, in order to cause frothing (foaming).
Air is then blown into the mixture so that the earthy impurities sink while the ore floats and mixes with the foam. The ore is finally recovered from the foam
PURE AND IMPURE SUBSTANCES: The following are the criteria for purity of chemical substances.
DENSITY: The density of a pure substance is definite and constant, while that of an impure substance higher than expected.
MELTING POINT: The melting point of a pure solid is sharp and definite. The presence of an impurity lowers the melting point of a substance, and spread its melting point over a wide range of temperatures

FREEZING POINT: The freezing point of a pure liquid is sharp and definite; the presence of an impurity lowers the freezing point.
BOILING POINT: The boiling point of a pure liquid is sharp and definite. An impurity raises the boiling point of a pure liquid.
TEST FOR PURITY
After separation of substances from mixtures, it is important to know if they are pure. A pure solid should melt at a constant temperature. A pure liquid should boil at a constant temperature. A pure dye should give only one spot on a chromatogram. The melting points or boiling points of pure substances are fixed. These temperatures change if impurities are present. To assess the purity of a substance its melting point (if it is a solid) or its boiling point (if it is liquid) is determined (if the value obtained agrees with that given in a book of data, then the substance is pure).
The apparatus below can be used to find the melting point of a solid.
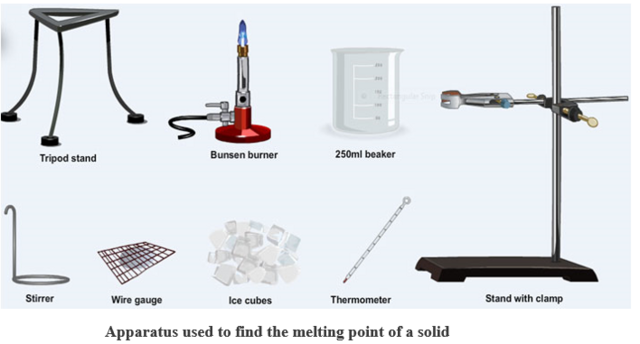
The melting point of a solid is the temperature at which it changes to liquid. The melting point tube is very thin- a capillary tube- and the substance under test must be finely powdered so that it can be packed into the capillary tube (melting point tube). The beaker containing the oil is heated slowly and the oil stirred vigorously. If the solid is pure it will all melt at a constant temperature. i.e. it will have a sharp melting point.
NOTE: If impurities are present the mixture will melt slowly over a range of temperatures below the melting point of the pure solid.
EVALUATION:
DETERMINATION OF THE BOILING POINT OF LIQUIDS
(a) Flammable liquids (b) In flammable liquids
The boiling point of a liquid is the temperature at which its vapour pressure equals atmospheric pressure.
The apparatus shown above can be used to find the boiling points of liquids.
A pure sample of liquid will boil at a fixed temperature and the reading on the thermometer will remain constant. If the light is not pure it will boil over a range of temperature above the boiling point of the pure liquid.
Impurities lower the melting point of a substance and raise its boiling point.
EVALUATION:
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 