The importance of electrochemical cells or galvanic cells lies in their ability to provide us with a portable source of electrical energy. We have already studied that indirect redox reaction is, primarily, the basis of all the electrochemical cells Quite often, we use the term battery to represent the arrangement of two or more galvanic cells connected in series.
However, in practice the redox reaction used should give the arrangement which fulfills the following requirements:
APPLICATIONS OF THE ELECTROCHEMICAL SERIES
Some of the important applications of the electrochemical series have been discussed as follows:
EMF o = E o cathode – E o anode
However, if the reaction taking place in the cell is also to be determined, the following steps are followed:
(a) Write reduction equations for both the electrodes along with their standard reduction potentials, separately.
(b) Balance each reaction with respect to the number of atoms of each kind and the electrical charges.
(c) Multiply the reactions by a suitable number so that the number of electrons involved in both the half reactions are equal.
(d) Subtract the equation for reaction having the lower standard reduction potential from the other reaction having higher standard reduction potential. The difference gives the standard EMF of cell.
The electrode having higher standard reduction potential acts as cathode and the other electrode acts as anode. Now let us solve some numerical problems to understand the applications of this method in calculating standard EMF.
Thus, we may conclude that the metals occupying higher positions in the electrochemical series are more reactive in displacing the other metals from the solutions of their salts. For example, zinc lies above copper in the series and hence, it can displace copper from copper sulphate solution. Copper cannot displace zinc from zinc sulphate solution because it lies below zinc in the series and hence, it is less reactive.
EMF o = E o cathode – E o anode
If EMF o comes out to be positive the given redox reaction will take place and if EMF0 comes out to be negative the given redox reaction will not take place.

For the above reaction to occur, the E o red of metal (M n+ M) must be lower than that of hydrogen. Hence, it can be concluded that metals which lie above hydrogen in the electromotive series can reduce H+ ions to hydrogen and hence, liberate hydrogen gas on reaction with dil acids. In other words, metals having negative reduction potentials can displace hydrogen from acids. For example, zinc (E o zn+2 / z n = -0.76 volt) lies above hydrogen in the series and hence, it can displace hydrogen from dilute acids, whereas copper (E0 eu•2teu = +0.34 volts) which is lying below hydrogen in the series cannot displace hydrogen from acids.
Fuel Cells
→ Fuel Cells
In recent years, the scientists have designed the cells which convert chemical energy of a fuel directly into electrical energy. Such cells are called fuel cells. These are the voltaic cells in which, the fuels such as H2, CO, CH4, C3H8, etc., are used to generate electrical energy without the intervention of thermal devices like boiler, turbines, etc.
The conventional method of conversion of chemical energy of a fuel into electrical energy involves combustion of a fuel to liberate heat. The heat energy so produced is used to generate steam for spinning the turbines which are coupled to electrical generators. This process is approximately 40% efficient.
Fuel cells are designed in such a way that the materials to be oxidised and reduced at the electrodes are stored outside the cell and are constantly supplied to the electrodes. In fact, fuel cell is a flow battery that continues to operate as long as the reactants from outside are fed into it. One of the most successful fuel cells uses the reaction of hydrogen and oxygen to form water and is known as H2O2 fuel cell. The H2-O2 fuel cell is also called Bacon cell after the name of its inventor and it had been used to power the Apollo space Missions. The water vapours produced during the reaction were condensed and added to drinking water supply for the astronauts. The experimental arrangement is shown in Fig. 33.13.
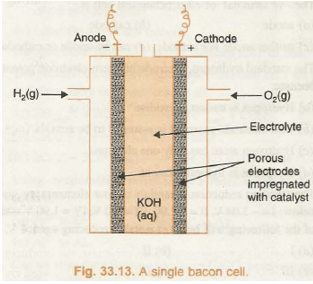
The cell consists of porous carbon electrodes which are impregnated with catalyst (Pt, Ag or CoO). Hydrogen and oxygen are bubbled through the electrodes into electrolyte which is an aqueous solution of NaOH or KOH. The electrode reactions are:
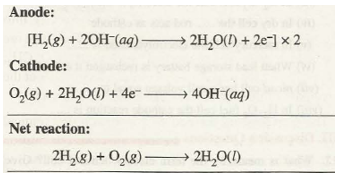
The cell consists of porous carbon electrodes which are impregnated with catalyst (Pt, Ag or CoO). Hydrogen and oxygen are bubbled through the electrodes into electrolyte which is an aqueous solution of NaOH or KOH. The electrode reactions are:
The cell runs continuously as long as the gases hydrogen and oxygen are supplied at the temperature 525 K and 50 atm. pressure.
ADVANTAGES OF FUEL CELLS
Some prominent advantages of fuel cells are being described as follows:
(i) Pollution Free Working. There is no harmful or objectionable product formed in fuel cells. Hence, they do not cause pollution problems.
(ii) High Efficiency. The efficiency of fuel cells is approximately 70-75%, which is much higher than the conventional cells.
(iii) Continuous Source of Energy. Unlike conventional batteries, energy can be obtained from the fuel cell continuously so long as the supply of fuel is maintained.
The H2/O2 cell has been used for generating electrical power in the Apollo space programmes.
Comparison Between Electrochemical Cell and Electrolytic Cell
Comparison Between Electrochemical Cell and Electrolytic Cell
We have learnt that there are two types of cells namely electrochemical cells and electrolytic cells. Electrochemical cell is a device which converts chemical energy into electrical energy. On the other hand, electrolytic cell is a device which converts electrical energy into chemical energy. The two cells also differ significantly with respect to the charges on the electrodes. For example, in electrochemical cell anode is negative whereas in electrolytic cell, the anode is positive. Similarly, cathode is positive in electrochemical cell whereas it is negative in the electrolytic cell. The main points of difference have been summed up as follows in Table 34.1.
| Galvanic Cell
1. In galvanic cell, electrical energy is produced.
2. In galvanic cell, reaction taking place is spontaneous.
3. The two half cells are set up in different containers and are connected through salt bridge or porous partition.
4. In galvanic cell, anode is negative and cathode is positive.
5. The electrons move from anode to cathode in external circuit. | Electrolytic Cell
1. In electrolytic cell, electrical energy is consumed.
2. In electrolytic cell, reaction taking place is nonspontaneous.
3. Both the electrodes are placed in the solution or molten electrolyte in the same container.
4. In electrolytic cell, the anode is positive and cathode is negative.
5. The electrons are supplied by the external source. They enter through cathode and come out through anode. |
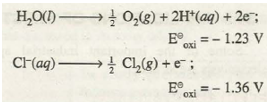
Although oxidation potential of H2O is more than that of CI- ions, yet during the electrolysis of concentrated solution of sodium chloride, the chloride ions oxidise in preference to H2O molecules at the anode giving Cl2 gas as the product.
The galvanic cells can be broadly classified into two categories, namely; primary cells and secondary cells.
PRIMARY CELLS
This type of cells become dead over a period of time and the chemical reaction stops. They cannot be recharged or used again. Some common examples are dry cell, mercury cell, etc.
(a) Dry Cell. It is a compact form of Leclanche cell known after its inventor, a French chemist, G Leclanche. In this cell, anode consists of zinc container while cathode is a graphite rod surrounded by powdered :MnO2 and carbon. The space between the electrodes is filled with the paste of NH4Cl and ZnCl2. The arrangement is shown in Fig. 33.9.
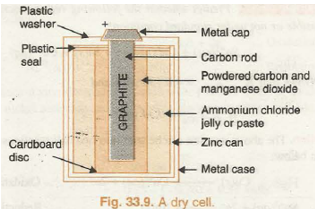
The reactions taking place at the electrodes are given in their simplified form as follows:
Cathode:
MnO2 + NH4+ e– à MnO(OH) + NH3
Anode:
Zn à Zn2+ + 2e–
The zinc ions (Zn2+) so produced combine with ammonia liberated in cathodic reaction to form diammine zinc (II) cation.
Zn2+ + 2NH3 à [Zn(NH3) 2]2+
Dry cells do not have long life as NH4Cl which is acidic, corrodes the zinc container even if the cell is not in use. The cell potential of dry cells lies in the range J. 25 V to 1. 5 V.
(b) Mercury Cell. It is miniature cell which finds a frequent use these days to supply energy for watches, video cameras, hearing aids and other compact devices. In mercury cell the anode is zinc-mercury amalgam and the cathode is a paste of mercury( II) oxide and carbon. Electrolyte is a moist paste of KOH-ZnO. The arrangement in its simple form is shown in Fig. 33.10.
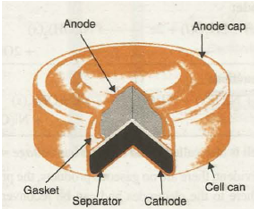
Fig. 33.10. Commonly used mercury cell. The reducing agent is zinc and the oxidising agent s mercury (‘I) oxide
The operating voltage for mercury cell is = 1.35 V and the cell reactions are as follows:
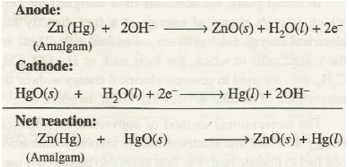
Such a cell has constant potential throughout its life
SECONDARY CELLS
This type of cells can be recharged by passing direct current through them and can be used again and again. Some examples are lead-storage battery, nickel-cadmium storage cell, etc. Let us study the working of lead storage cell.
(a) Lead-storage Battery. It is the most frequently used battery in automobiles. It consists of six voltaic cells connected in series. In each cell anode is made of spongy lead and cathode ‘is a grid of lead packed with lead dioxide (PbO2). The electrolyte is the aqueous solution of H2SO 4 which is 38% by mass. The reactions taking place in this type of cell can be represented as:
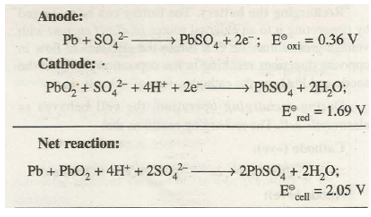
During the working of the cell, the concentration of H2SO4 decreases as sulphate ions are consumed to form PbS04. The PbSO4 precipitates and partially gets coated on both the electrodes and water formed dilutes the sulphuric acid. With the decrease in the concentration of H2SO4 the density of the solution also decreases. The condition of the battery can be easily checked by measuring the density of the solution .
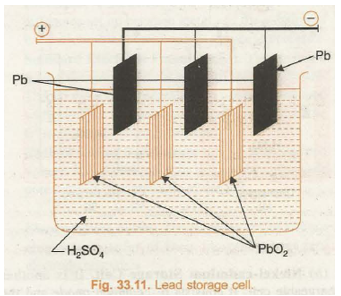
To enhance the output of the cell, the anode and cathode plates are arranged in alternating manner and they are separated by sheets of insulating material. The anode and cathode plates are separately connected to each other so as to increase the electrode area in contact with electrolyte solution. This increases current delivering capacity of the cell. The groups of electrodes constitute one cell are shown in Fig. 33.11. The cells are further connected in series so as to increase the voltage of the battery. In 6 volts battery there are 3 cells and in 12 volts battery there are 6 cells.
Recharging the battery. The battery can be recharged by connecting it to an external source of direct current with voltage greater than 12 V. It forces the electrons to flow in opposite directions resulting in the deposition of Pb on the anode and Pb02 on the cathode.
During recharging operation, the cell behaves as electrolytic cell. The recharging reactions are
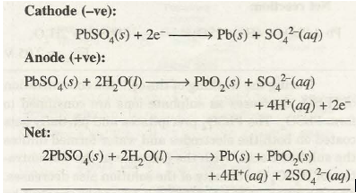
Such an operation becomes possible because PbSO4 formed during discharge operation is solid and sticks to the electrodes. Therefore, it is in a position to lose or gain electrons during electrolysis.
The discharging and recharging of lead storage battery has been shown in Figs. 33.12 (a) and 33.12 (b) respectively.
In an automobile, the battery is discharged when the engine is started. While running, the engine powers the alternator which produces electrical energy sufficient enough to recharge the battery. Thus, battery is constantly recharged as long as the automobile is being driven.
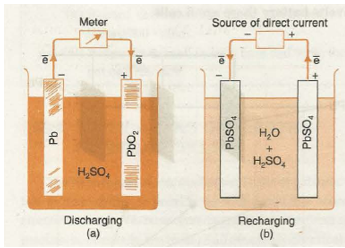
Fig. 33.12. Discharging and recharging of lead storage cell
(b) Nickel-cadmium Storage Cell. It is another rechargeable cell. It consists of cadmium anode and the cathode is made of a metal grid containing nickel (IV) oxide.
These are immersed in KOH solution. The reactions occurring are:
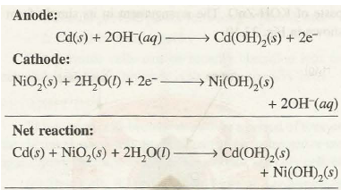
The cell is also called nicad cell and has voltage =1.4 V. As is evident, there are no gaseous products, the products formed adhere to the electrodes and can be reconverted by recharging process. This cell is becoming more popular these days and finds use in electronic watches and calculators.
EVALUATION
1.What are the applications of electrochemical cells.
2.Describe the structure of i.the Daniel cell ii.the Leclanche’ cell and give the chemical reactions taking place in them respectively.
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 