The passage of electricity through the electrolytes in their molten or dissolved state can cause chemical changes under suitable conditions. For example, the passage of electricity through the acidified water results in the formation of hydrogen and oxygen gases. The process of chemical decomposition of the electrolyte by he passage of electricity through its molten or dissolved state is called electrolysis.
ELECTROL YTIC CELL
The device in which the process of electrolysis is carried 1ut is called electrolytic cell. It consists of:
(i) Electrolytic tank, which is made of some nonconducting materials like glass, wood or bakelite.
(ii) Electrolyte in its dissolved state or molten state.
(iii) Source of electricity; an electrochemical cell or battery
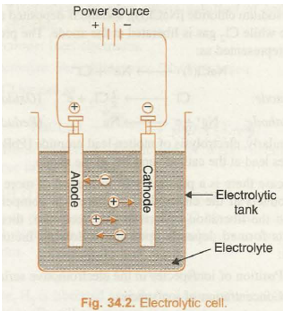
(iv) Two metallic rods, suspended in the electrolyte and connected to the battery through conducting wires. These rods are called electrodes. The electrode connected to the negative terminal of battery is called cathode while the other one which is connected to the positive terminal is called anode. The apparatus used to constitute electrolytic cell has been shown in Fig. 34.2.
Mechanism and Criteria of Product Formation in Electrolytic Cell
Mechanism and Criteria of Product Formation in Electrolytic Cells
The process of electrolysis can be explained on the basis of the theory of ionisation. When an electrolyte is dissolved in water, it splits up into charged particles called ions. The positively charged ions are called cations while the negatively charged ions are called anions. The ions are free to move about in aqueous solution. When electric current is passed through the solution, the ions respond to the applied potential difference and their movement is directed towards the oppositely charged electrodes. The cations move towards the negatively charged electrode while anions move towards the positively charged electrode. The formation of products at the respective electrodes is due to oxidation (loss of electrons) at the anode and reduction (gain of electrons) at the cathode.
For example, when electricity is passed through the molten sodium chloride [NaCl)], sodium is deposited at the cathode while C~ gas is liberated at the anode. The process can be represented as:
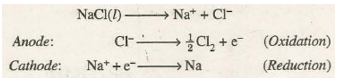
Similarly, electrolysis of molten lead bromide [PbBr2(l)] produces lead at the cathode and Br2 at the anode.
In case there is a possibility of formation of more than one products at the electrodes, or there is a competition between the liberation of ions at the electrodes, then the products formed depends upon the following factors in general:
One of the dominant factors which control the criterion of product formation at the electrodes is the values of electrode potentials of the species. Let us understand it as follows:
(a) At the Cathode. Cathode involves reduction process at its surface. Therefore, for the different competing reduction processes, the one with higher reduction potential, will preferably take place. For example, during the electrolysis of aqueous solution of sodium chloride there is possibility of following reactions at the cathode:
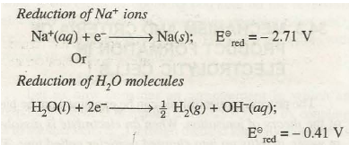
The reduction of water will preferably take place at the cathode because E red of water is higher. Hence, the product of electrolysis of aqueous solution of NaCl at the cathode will be H2 gas instead of Na(s).
Similarly, during electrolysis of aqueous solution of Copper (II) tetraoxosulphate (VI) reduction of Cu2+ ions will take place at the cathode in preference to the reduction of 0 molecules because E2 is greater than E2
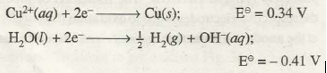
(b) At the Anode. Anode involves oxidation process at its surface. Therefore, for different competing oxidation processes, the one with higher oxidation potential (or lower reduction potential) will preferably occur. For example, if we carry out electrolysis of aqueous solution of copper(II) tetraoxosulphate(VI), the competing oxidation processes at the anode are as follows:
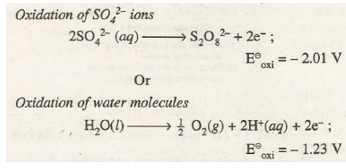
As oxidation potential of water is higher, the product formed at the anode will be O2 gas instead of S2O8O 2– ion.
Similarly, if the electrolysis of copper sulphate solution is carried out using copper electrodes, then the process occurring at the anode will be oxidation of copper atoms to copper ions instead of oxidation of water because oxidation potential of Cu is higher.
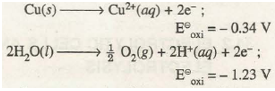
Thus, in such a case copper from anode will go on dissolving into solution as Cu2+ ions while Cu2+ ions from solution will go on depositing at the cathode as copper atoms.
The above discussion leads us to a general conclusion that for different competing reactions at the electrodes:
It may be noted that in some cases the unexpected results are obtained due to overvoltage*. For example, let us compare the oxidation potentials of CI- ion and water
ELECTROLYSIS OF SPECIFIED ELECTROLYTES
In the light of above discussion let us discuss some examples of electrolytic reactions. Before we take up the actual examples, it is quite important to know about inert electrodes and active electrodes.
Inert electrodes are those electrode which do not gain or lose electrons during the process. Two common examples are carbon (graphite) electrode and platinum electrodes.
Active electrodes are those electrodes which themselves participate in the gain or loss of electrons.
Electrodes: Inert electrodes
Electrolyte: Molten lead (II) bromide Molten lead (II) bromide ionises as
Reaction at anode (oxidation of Br-)
Electrodes: Inert electrodes
Electrolyte: Sodium chloride dissolved in water solvent.
NaCl ionises in aqueous solution as
NaCl (l) à Na + + Cl –
Cathode reaction: Reduction of H2O occurs in preference to Na+ ions as Na+ is much higher in electromotive series
2H2O (l) – à H2 (g) + 2OH (aq)
Anode reaction: Oxidation of CI- ions occur in preference to H2O because of higher concentration of CI- ions and overvoltage
2Cl –(aq) à Cl2 (g) + 2e –
Thus, Cl2 gas is produced at anode and H2 gas is liberated at cathode. The pH of solution increases due to increase in the concentration of OH ions in solution.
Electrode: Inert electrodes.
Electrolyte: Very dilute brine (NaCl) solution.
NaCl ionises as
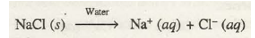
Cathode reaction: Reduction of water occurs in preference to Na+ ions
4H2O (l) + 4e – à 2H2 (g) + 4 OH – (aq)
Here, H2 is liberated at cathode whereas O2 is liberated at the anode. The pH of solution does not change due to equal consumption of H+ and OH ions. However, concentration of NaCl gradually increase due to decomposition of water.
Electrodes: Inert electrodes (Pt).
Electrolyte: CuSO 4 dissolved in water CuSO 4 ionises in aqueous solution as
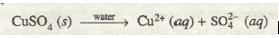
Cathode reaction: Cu2+ ions undergo reduction in preference ~0 molecules
eu2+ (aq) + 2e– à7 Cu (s)
Anode reaction: Cu atoms from the copper electrode get oxidised in preference to ~0 because Cu lies below Hp in activity series.
Cu (s) à Cu2+ (aq)
Thus, copper dissolves from anode and get deposited at cathode. Mass of cathode increases and that of anode decreases. Blue colour of the solution does not fade because concentration of Cu2+ ions in solution remains unaltered. The pH of the solution also does not change as the electrolysis proceeds.
Applications of Electrolysis
Some of the important industrial applications of electrolysis are as FOLLOWS.
ELECTROMETALLURGY
Many of the highly reactive metals such as metal of group 1 or group 2 are extracted from their ores by electrolysis of their molten ores. This is because chemical reduction of their compounds .is either chemically not viable or highly expensive. Metals like sodium and magnesium, are manufactured by the electrolysis of their molten chlorides. Pure aluminium is obtained at Valco Industry from a solution of its oxide in molten cryolite. Details of this extraction and that of some other metals is discussed in later units.
ELECTROREFINING OF METALS
Refining of many of the crude metals such as copper, silver; aluminium, etc. is carried out by the process of electrolysis. In this. process, the block of crude metal is made anode while a thin sheet of pure metal is made cathode and the electrolyte is aqueous solution of some salt of the metal. As the electrolysis proceeds metal from anode dissolves whereas it gets deposited in pure form at cathode. The impurities settle down just below anode in the form of anode sludge or anode mud.
MANUFACTURE OF CHEMICAL SUBSTANCES
Electrolysis is used in the manufacture of some important substances such as hydrogen, chlorine, sodium hydroxide, sodium oxochlorate (I) and oxygen.
Electroplating
It is another important industrial application of electrolysis. Electroplating is an art of coating a layer of costlier metal like gold, silver, etc. over the cheaper metal like iron. The purpose of electroplating is
The principle of electroplating is similar to that of purification of metals by electrolysis. The article to be plated, is thoroughly cleaned with aq H2SO 4 and washed with distilled water. It is then made the cathode of the electrolytic cell. The anode is pure sheet of metal to be coated or plated. The electrolyte is a solution of a salt of the metal to be plated. During electrolysis, the metal to be electroplated is transferred from the anode to the cathode.
APPLICATION OF ELECTROLYSIS
Electrolysis has several uses in industry. Its main application has been in the fields of manufacture of chemicals and in the purification of metals for which other purification methods prove either too difficult or highly expensive to apply. Some applications of electrolysis are as discussed below:
Reactive metals exist as compounds, so it is difficult to extract them from their compounds. Extraction of metals such as sodium, potassium, magnesium and calcium necessarily requires an electrolytic process. For example, electrolytic method is used to extract sodium from molten sodium chloride:
2NaCl(l) → 2Na(l) + Cl2(g)
Some metals can be purified by means of electrolysis. This process is used in industry to purify copper, which must be very pure 99.9% for electrical wiring. Copper made by roasting the sulphide ore is about 99.5% pure (so it has an impurity level of 0.5%). This level of impurity cuts down electrical conductivity significantly.
This is how the electrolytic purification (refining) process is carried out:
The anode is made of a large block of impure copper. The cathode is a thin sheet of pure copper. The electrolyte is copper (II) sulphate solution.
During the refining process, the copper atoms of the impure block become ions (the anode dissolves).
Cu → Cu2+ + 2e–
The ions from the solution become atoms.
Cu2+ + 2e– → Cu(s)
They stick onto the cathode. A layer of pure copper builds up on the cathode. As electrolysis takes place, the cathode gains mass as copper is deposited on it. As a result, the cathode gets smaller while the cathode gets bigger as electrolysis proceeds. Eventually the whole cathode dissolves.
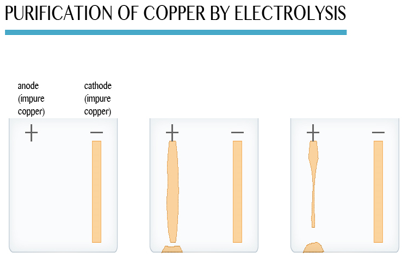
Only pure copper sticks to the cathode. Most impurities fall to the bottom of the electrolytic cell. They form a solid material (anode sludge or slime) which contains small quantities of precious metals such as silver, gold and platinum. The precious metals recovered from the slime are purified and sold.
The electrolysis of a concentrated sodium chloride solution (brine) is an important industrial process. The electrolysis of brine produces hydrogen, chlorine and sodium hydroxide. The electrolysis is carried out in an apparatus called Down’s cell.
The diagram below shows one of several types of cell that are used. The cell is sometimes referred to as the membrane cell.
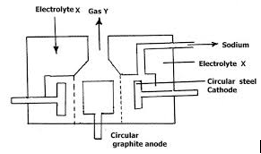
The anode is made of titanium and the cathode of nickel. Anion-exchange up the middle lets sodium ions through but keeps the gases apart. The sodium ions move freely to the cathode.
At the cathode: hydrogen bubbles off:
2H+ + 2e– → H2(g)
At the anode: chlorine bubbles off:
2Cl– → Cl2 + 2e–
Na+ and OH– ions are left behind, which means a solution of sodium hydroxide forms. Some is evaporated to a more concentrated solution, and some evaporated completely to give solid sodium hydroxide.
The products of this electrolysis have a wide range of uses.
Hydrogen is used to make ammonia and in the manufacture of margarine.
Chlorine is used to kill bacteria in swimming pools and domestic water supplies. It is also used to make plastics.
Sodium hydroxide is used to make soap, paper, bleach, and rayon fibres.
The products also have several other uses not mentioned here.
Electroplating is the coating of a metal with a layer of another metal by means of electrolysis. Electrolysis can be used to coat a thin layer of a less reactive metal onto a more reactive metal. The thin layer of less reactive metal will provide protection from corrosion for the more reactive metal underneath. It may also make the product more attractive.
The object to be coated should be made the cathode and the coating material should be the electrolyte. The most commonly used metals for electroplating are copper, chromium, silver and tin.
Steel can be electroplated with chromium or tin. This prevents the steel from rusting and gives it a shiny, silver finish. This is also the idea behind chromium-plating articles such as car bumpers, kettles, bath taps, etc. Chromium does not corrode, it is a hard metal that resists scratching and wear, and can also be polished to give an attractive finish.
Nickel can be electroplated with silver. This will make nickel more attractive.
The diagram below shows how a steel jug is electroplated with silver. The jug becomes the cathode of an electrolytic cell. The anode is made of silver. The electrolyte is a solution of a silver compound, for example silver nitrate.
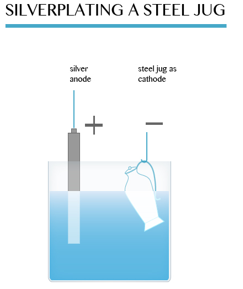
At the anode: The silver dissolves, forming ions in solution:
Ag → Ag+ + e–
At the cathode: The silver ions receive electrons, forming a coat of silver on the jug:
Ag+ + e– → Ag (s)
When the layer of silver is thick enough, the jug is removed.
In general, to electroplate any object with metal M, the set up is:
Cathode – object to be electroplated
Anode – metal M
Electrolyte – solution of a soluble compound of M
Although aluminium is quite reactive, corrosion in air is not a problem. This is because a thin layer of aluminium oxide quickly forms and acts as a barrier to oxygen. The layer can be made thicker, to give a more protection by a process called anodizing.
The aluminium is used as the anode of a cell. Dilute sulphuric acid is the electrolyte. When this acid is electrolysed, as you learned early, oxygen forms at the anode.
4OH–→2H2O(l) + O2(g) + 4e–
The oxygen liberated at the aluminium anode reacts with aluminium to form a protective layer of the oxide on the metal:
4Al(s) + 3O2(g) → 2Al2O3(g)
The anodized aluminum absorbs dyes and pigments easily.It is used for a whole range of things including masts for yachts and windsurfing boards, facings for buildings, and window frames.
Hydrogen may become a much more important fuel in the future. It can be produced by the electrolysis of acidified water. Pure water is a very poor conductor of electricity. However, it can be made to decompose if some sulphuric acid is added.
Sulphuric acid, when dissolved in water, forms ions:
H2SO4(aq) → 2H+(aq) + SO42-(aq)
Water ionizes thus: H2O⇔H+ + OH–
Therefore, a dilute solution of the acid contains H+ ions from water and acid, OH– ions from the water and SO42- ions from the acid.
| At cathode | At anode |
| Ions: H+ | OH– and SO42- |
| Reaction: 2H+ +2e– →H2(g) | 4OH–→2H2O(l)+O2(g)+4e– |
| Product: Hydrogen is liberated | Oxygen is liberated. |
A Hofmann voltammeter shown below can be used to keep the gases produced separate. The gas collected above the cathode is hydrogen. Oxygen collects at the anode. The ratio of the volume is approximately 2:1. Effectively this is the electrolysis of water.
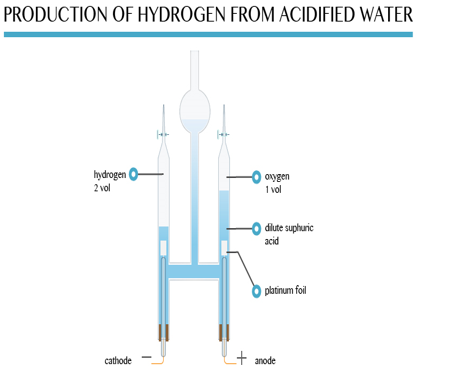
In the future, hydrogen from the electrolysis of water may be piped into homes and used as a fuel for cooking and heating. It will also be used in batteries called fuel cells to power homes and electric cars.
EVALUATION
2. Name the electrode
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 