When an electric current is passed through an electrolyte solution, the ions of the electrolyte undergo chemical changes at the respective electrodes. The chemical reaction carried out by passing electricity is called electrolysis.
it is important that we familiarize ourselves with different terms that we are going to use to explain different phenomena. It is crucial that the definitions and meanings of these terms be understood at the outset in order that concepts defined in this chapter are easily and clearly apprehended. These terms are given hereunder:
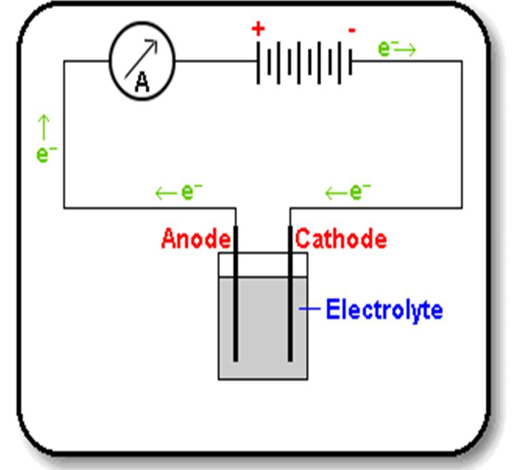
The electrolytic cell (voltameter)
The apparatus in which electrolysis is carried out is called electrolytic cell. A battery supplies the direct current. Graphite electrodes carry the current into and out of the liquid electrolyte. Graphite is chosen because it is quite unreactive (inert). It will not react with the electrolyte or with products of electrolysis. Electrons flow from the negative terminal (cathode) of the battery around the circuit and back to the positive terminal (anode). In the electrolyte it is the ions that move to carry the current.
6.2. THE MECHANISM OF ELECTROLYSIS
The conductivity of ionic compounds is explained by the fact that ions move in a particular direction in an electric field. This can be shown in experiments with coloured salts. For example, copper (II) chromate (VI) (CuCrO4) dissolves in water to give a green solution. This solution is placed in a U-tube. A colourless solution of dilute hydrochloric acid (HCl) is then layered on top of the salt solution in each arm. Graphite rods are fitted as shown in figure 13.3. These rods (electrodes) carry the current into and out of the solution.
After passing the current for a short time, the solution around the cathode becomes blue. Around the anode, the solution becomes yellow. These colours are produced by the movement (migration) of the ions in the salt. The positive copper ions (Cu2+) are blue in solution. They are attracted to the cathode (negative electrode). The negative chromate ions (CrO42-) are yellow in solution. They are attracted to the anode (the positive electrode). The use of coloured ions in solution has shown the direction that positive and negative ions move in an electric field. Always positive ions (cations) move to the cathode and negative ions (anions) move to the anode
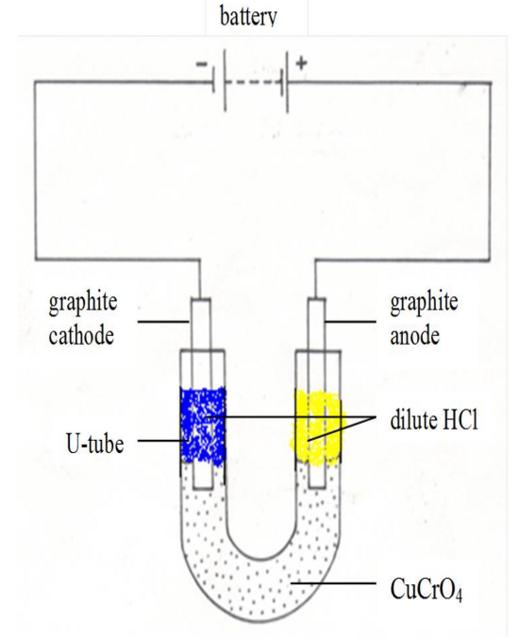
The movement of ions in solution
PREFERENTIAL (SELECTIVE) DISCHARGE OF IONS
When a salt such as sodium chloride is dissolved in water, its ions are set free to move. So the solution can be electrolysed. Since the salts, alkalis and acids are dissolved in water, most of the solutions are aqueous. There is then a complication in electrolysis of such substances in aqueous form. This is because the water used to dissolve them also do ionize partially (it is a weak electrolyte)
H2O⇔ H+ + OH–
Then at each electrode, we get more than one ion for discharge, but only one is supposed to be discharged. Take an example of electrolysis of copper (II) sulphate solution using platinum electrodes. By ionic theory, the solution ionizes thus:
CuSO4 (aq) → Cu2+ (aq) + SO42-(aq) (strong electrolyte)
H2O⇔ H+ + OH– (weak electrolyte)
During electrolysis, Cu2+ and H+ ions move to the cathode while SO42-and OH– ions move to the anode.
| Cathode | Anode |
| Cu2+ | SO42- |
| H+ | OH– |
In situations like this, the order of discharge of the ions at the electrode will depend on:
| Cations | Anions
| ||
| K+ | ease of discharge increases | SO42- | ease of discharge increases |
| Ca2+ | NO3– | ||
| Na+ | Cl– | ||
| Mg2+ | Br– | ||
| Al3+ | I– | ||
| Zn2+ | OH– | ||
| Fe2+ | |||
| Pb2+ | |||
| H+ | |||
| Cu2+ | |||
| Ag+ |
The arrangement of ions above is the same as that of the electrochemical series. If all other factors are constant, any ion will be discharged from solution in preference to those above it. For example, in sodium hydroxide solution, containing H+ (from water) and Na+ (from the salt), H+ discharges in preference to Na+.
In copper sulphate solution, containing OH– (from water) and SO42- as negative ions, OH– is discharged in preference to SO42-.
| Cathode |
| Anode |
| Cu2+ | SO42- | |
| H+ | OH– | |
| Cu2+ + 2e– → Cu(s) copper is discharged (loses its charge) |
| 4OH–→2H2O(l)+O2(g)+4e– hydroxyl ion is discharged |
In other words, we can say that the reaction that occurs at the cathode is reduction (electron gain) and that which occurs at the anode is oxidation (electron loss).
Result
Take an example of electrolysis of sodium chloride using platinum electrodes when in:
NaCl → Na+ + Cl–
H2O⇔H+ + OH–
During electrolysis:
| Cathode | Anode |
| Na+ and H+ | Cl– and OH– |
| H+ ions are discharged in preference to Na+ ions: 2H+ + 2e– → H2(g)
| OH– ions are discharged in preference to Cl– ions: 4OH–→2H2O(l) +O2(g)+4e–
|
Result
NaCl → Na+ + Cl–
H2O⇔H+ + OH–
| Cathode | Anode |
| Na+and H+
H+ ions are discharged in preference to Na+ ions since Na+ and H+ are very far from each other in e. c.s 2H+ + 2e– → H2(g) | Cl– and OH–
Cl– ions are discharged in preference to OH– ions since Cl– and OH– ions are very close to each other in the e. c. s (and because there are more Cl– ions in the solution) 2Cl– → Cl2(g) + 2e– |
Result
Na+(aq) + OH–(aq) → NaOH(aq)
NaCl → Na+ + Cl–
During electrolysis:
| Cathode | Anode |
| Na+ + e– → Na(l)
| 2Cl– → Cl2(g) + 2e–
|
Result
Example 1
H2SO4 → 2H+ + SO42-
H2O⇔H+ + OH–
| Cathode | Anode |
| 2H+ + 2e– → H2(g) | SO42- and OH– 4OH–→2H2O(l)+O2(g)+4e– |
Result
H2SO4 → 2H+ + SO42-
H2O⇔H+ + OH–
| Cathode | Anode |
| 2H+ + 2e– → H2(g)
| SO42- and OH–
|
| 4OH–→2H2O(l) + O2(g) + 4e– | |
|
| C + O2 → CO2(g)
|
Result
Example 2
Electrolysis of copper (II) sulphate using:
CuSO4 → Cu2+ + SO42-
H2O⇔H+ + OH–
| Cathode | Anode |
| H+ and Cu2+ | SO42- and OH– |
| Cu2+ + 2e– → Cu(s)
| 4OH–→2H2O(l) + O2(g) + 4e-
|
Result
2H+ + SO42- → H2SO4
CuSO4 → Cu2+ + SO42-
H2O⇔H+ + OH–
| Cathode | Anode |
| H+ and Cu2+ |
|
| Cu2+ + 2e– → Cu(s) |
|
|
The SO42- and OH– ions move to the anode and there are possibilities of one of these being discharged. However, these ions are not discharged but remain in solution. Instead, copper atoms of the anode lose electrons and go into solution as copper ions.
Cu → Cu2+ + 2e–
Result
EVALUATION
1.Define Electrolysis
Ii Define the following terms
I.anode ii. cathode ii i. anoins iv. cations v.ion vi. electrode
ii State 3 factors that affect the preferential discharge of ions.
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 