Amino acids are the basic structural units of proteins. Amino acids are organic compounds which contain both an amino group and a carboxyl group, that is, any of a group of organic molecules that consist of a basic amino group (−NH2), an acidic carboxyl group (−COOH). Each molecule contains a central carbon (C) atom, termed the α-carbon, to which both an amino and a carboxyl group are attached. The remaining two bonds of the α-carbon atom are generally satisfied by a hydrogen (H) atom and the R group. The formula of a general amino acid is:
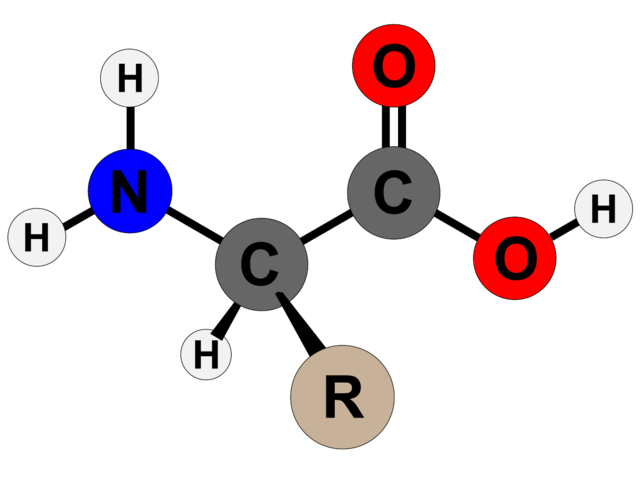
The amino acids differ from each other in the particular chemical structure of their R group.
There are 20 naturally occurring amino acids of biological importance. The human body can synthesize all of the amino acids necessary to build proteins except for the ten called the “essential amino acids”. They can be supplied by a combination of cereal grains (wheat, corn, rice, etc.) and legumes (beans, peanuts, etc.). The 10 amino acids that we can produce are alanine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine and tyrosine. Tyrosine is produced from phenylalanine, so if the diet is deficient in phenylalanine, tyrosine will be required as well. The essential amino acids (that we cannot produce internally) are arginine (required for the young, but not for adults), histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine. These amino acids are required in the diet. Plants, of course, must be able to make all the amino acids. Humans, on the other hand, do not have all the enzymes required for the biosynthesis of all of the amino acids.”
Peptides
Peptides are amides formed by the interaction between amino groups and carboxyl groups. The bond joining the α – amino group of one amino acid and the α – carboxyl group of another amino acid is called peptide bond. Two amino acids react to form a dipeptide, three to form a tripeptide etc. If a numbe of amino acids are linked by peptide bonnds, a polypeptide is formed. A polypeptide chain has an amino acid end and a carboxyl end. It consists of a regular repeating part or main chain and a variable part. or side chain.
Proteins
Proteins are highly complex substance that is present in all living organisms. Proteins are of great nutritional value and are directly involved in the chemical processes essential for life. A protein molecule is very large compared with molecules of sugar or salt and consists of many amino acids joined together to form long chains, much as beads are arranged on a string. There are about 20 different amino acids that occur naturally in proteins. Proteins of similar function have similar amino acid composition and sequence. When proteins are eaten, they are hydrolysed in the stomach to give a large variety of amino acids, which are then carried by the blood stream to all parts of the body. Some are used to build cell proteins in the growing body or repair damaged cells. Proteins are made up of polypeptide chains. The amino acid sequence in the polypeptide chain of a protein is specified by genes.
Glycine and alanine can combine together with the elimination of a molecule of water to produce a dipeptide.

In each case, the linkage shown in blue in the structure of the dipeptide is known as a peptide link.
If you joined three amino acids together, you would get a tripeptide. If you joined lots and lots together (as in a protein chain), you get a polypeptide.
Occurrence
Proteins are found in all living systems as structural components and as biologically important substances such as hormones, enzymes and pigments. Proteins in our food can be divided into first class and second class proteins. First class proteins contain essential amino acids and they are mainly of animal origin. Examples are lean meat, fish, eggs, milk and cheese. Second class proteins are mainly vegetable proteins such as beans and peas.
The relationship between amino acid side chains and protein conformation
The defining feature of an amino acid is its side chain (at top, blue circle; below, all colored circles). When connected together by a series of peptide bonds, amino acids form a polypeptide, another word for protein. The polypeptide will then fold into a specific conformation depending on the interactions (dashed lines) between its amino acid side chains.
Some important proteins are:
The hydrolysis is catalysed by an acid or base. The amino acids obtained on hydrolysis can be separated and identified by using paper chromatography.
Test for Proteins
Properties
Denaturation – Proteins usually form colloidal solutions. When such solutions are heated, the proteins precipitate or coagulate. This is due to the irreversible changes in the molecular shapes of the proteins, and the proteins are said to have been denatured. Proteins are easily denatured by
Hydrolysis
Proteins can be hydrolyzed to give amino acids by boiling them with solutions of hydrochloric acid. Hydrolysis is carried out using suitable enzymes.
ASSESSMENT (POST ANSWERS BELOW USING THE BOX)
Explain the denaturation of protein.
Mention 3 examples of proteins
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 