PHOTOELECTRIC EFFECT
INTRODUCTION
In 1887, photoelectric effect was invented by the scientist H. Hertz. When we passing a light into a material, the material should emit an electrons. This effect is called as Photoelectric effect. Some of the rays produced in Photoelectric Effect are x-rays, ultraviolet rays and γ-rays.
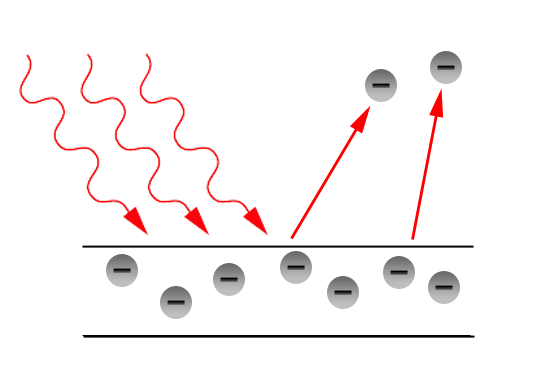
Einstein’s says that every photon or a quantum of light has an energy value hν. Electrons are located inside the surface of the metal. There may be some amount of work W should be required for an electron to come out of the metal. If the transferred energy is high comparing the internal attractions then the electrons gets liberated. In this the one photon can release a one electron. This is nothing but the Photoelectric Effect. Let us study more about the Photoelectric effect in this section.
It is the phenomenon of emission of electrons from the surface of metals when the radiations of suitable frequency and suitable wavelength fall on the surface of the metal.
The following parameters are related to Photoelectric effect:
Photoelectric Current
Stopping Potential
Threshold Energy
Work Function
The emitted electrons are called photo electrons and the current so produced is called as the Photoelectric Current. The intensities of photo electrons vary with material.
The Stopping Potential is the potential difference applied to stop the electrons from being ejected from the surface when the light falls on it.
The Threshold Frequency is defined as the minimum frequency of incident light required for the photo electric emission. It is denoted by ν.
Work Function is the minimum amount of energy necessary for the photo electric emission to start. It is denoted by ϕ0.
Photoelectric Effect Equation
The Einstein equation describes the phenomena of photoelectric effect but the validity of this equation is only up to two regions, one is visible light and second is for ultraviolet light.
The energy of photon is equal to energy required to remove electron + kinetic energy of the emitted electron.
E = W + K.E.
where E = Energy of photon
W = Work function
K.E = Kinetic Energy.
Energy of photon, E = h ν …………………….. (a)
Where h = Planck’s constant
ν = frequency of photon incident light or
So h ν = W + K.E …………………………… (b)
The work function W is defined as the minimum energy needed to remove an electron from the surface of a given metal. It is equal to h ν0.
K.E= This is the kinetic energy (maximum) of emitted electrons.
K.E = 1/2 mv2.
Here ν0 is the threshold frequency.
m = rest mass of the emitted electron
v = The speed or velocity of the emitted electron.
Work function or threshold energy ( ϕ ):
The minimum energy of incident photon below which no ejection of photon electron from a metal surface will take place is known as work function of threshold energy for that metal.
ϕ = h ν0 = hC/λ0
Work function is the characteristic of a given metal. If E is the Energy of incident photon then
Photoelectric Effect Experiment
The Phenomenon was discovered by Hertz and was experimentally proved by Thomson and Millikan. Einstein added his photoelectric equation to support the phenomenon. The phenomenon is basically about light energy (photo) converting into electrical energy.

The Apparatus consists of an evacuated quartz tube having photosensitive plate called emitter A and collector B. Plate A is connected to the negative terminal and plate B is connected to the positive terminal of a battery via rheostatR. The potential difference between the plates AB can be adjusted by changing the value of the rheostat. When light of suitable wavelength is incident on the plate A then electrons are emitted and reaches plate B, thus measurable current flows through the circuit.
Factors on which Photoelectric effect depends :
The amount of current flow (number of electrons) and the kinetic energy of the emitted electrons depend upon :

For a given photo metal if the frequency and applied voltage is kept constant with increasing intensity then we observe that photo current increases with increases in intensity with out change in the stopping potential.
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 