Nitrogen has 5 electrons in its valence shell. It has a valency of 3 with respect to hydrogen and a valency up to 5 with respect to oxygen. So, it can combine with various elements to form many compounds.
The well known compounds of nitrogen are
The Synthesis of ammonia – The Haber Process of Nitrogen Fixation
The initial yield is 6%-8%, but unreacted gases are recycled to raise this to nearer 100%
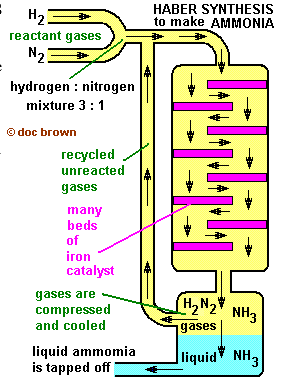
The summary of the formation of ammonia
nitrogen + hydrogen ammonia
N2(g) + 3H2(g) 2NH3(g) (plus 92 kJ heat energy, exothermic)
Ammonia formation is favouredby …
Rule 1. Lowering the temperature, because it is an exothermic reaction, but this may make it too slow, compromise required.
Rule 2. Increasing pressure, because there is a reduction in the molecules of gas, but the higher the pressure, the more costly the engineering.
Rule 4. An iron catalyst speeds up the reaction, but has no effect on the % ammonia in the reacted mixture exiting the reactor chamber in the chemical plant of a Haber synthesis process.
The Uses of Ammonia
Ammonia is used to make ammonium salts (mostly artificial fertilisers) and is used in the chemical industry in the manufacture of explosives, nitric acid and nitrates, pharmaceutical products and plastics.
(a) Ammonia is used to manufacture nitric acid
(b) Ammonia is used to manufacture ‘artificial’ nitrogenous fertilisers
(i) ammonia + hydrochloric acid ==> ammonium chloride
NH3 + HCl ==> NH4Cl
NH3(aq) + HCl(aq) ==> NH4Cl(aq)
(ii) ammonia + sulphuric acid ==> ammonium sulphate
2NH3 + H2SO4 ==> (NH4)2SO4
2NH3(aq) + H2SO4(aq)==> (NH4)2SO4(aq)
(iii) ammonia + nitric acid ==> ammonium nitrate
NH3 + HNO3 ==> NH4NO3
NH3(aq) + HNO3(aq)==> NH4NO3(aq)
Reactions (ii) and (iii) are used in fertiliser production, as is reaction (iv)
ammonia + phosphoric acid ==> ammonium phosphate
EVALUATION
1.Describe the following oxides of Nitrogen.
N2O4, NO2 , N2O5 , NO
2.Which of the oxides of nitrogen is a neutral oxide?
a.N2O4 b.NO2 C.N2O5 d.NO
3.Describe the Haber process
4.How is trioxonitrate v acid produced from ammonia?
5.Describe the brown ring experiment.
post your answer on the forum for review
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 