There are six elements in Group VIIA, the next-to-last column of the periodic table. As expected, these elements have certain properties in common. They all form diatomic molecules (H2, F2, Cl2, Br2, I2, and At2), for example, and they all form negatively charged ions (H–, F–, Cl–, Br–, I–, and At–).
When the chemistry of these elements is discussed, hydrogen is separated from the others and astatine is ignored because it is radioactive. (The most stable isotopes of astatine have half-lives of less than a minute. As a result, the largest samples of astatine compounds studied to date have been less than 50 ng.) Discussions of the chemistry of the elements in Group VIIA therefore focus on four elements: fluorine, chlorine, bromine, and iodine. These elements are called the halogens (from the Greek hals, “salt,” and gennan, “to form or generate”) because they are literally the salt formers.
None of the halogens can be found in nature in their elemental form. They are invariably found as salts of the halide ions (F–, Cl–, Br–, and I–). Fluoride ions are found in minerals such as fluorite (CaF2) and cryolite (Na3AlF6). Chloride ions are found in rock salt (NaCl), the oceans, which are roughly 2% Cl– ion by weight, and in lakes that have a high salt content, such as the Great Salt Lake in Utah, which is 9% Cl– ion by weight. Both bromide and iodide ions are found at low concentrations in the oceans, as well as in brine wells in Louisiana, California, and Michigan.
The Halogens in their Elemental Form
Fluorine (F2), a highly toxic, colorless gas, is the most reactive element known so reactive that asbestos, water, and silicon burst into flame in its presence. It is so reactive it even forms compounds with Kr, Xe, and Rn, elements that were once thought to be inert. Fluorine is such a powerful oxidizing agent that it can coax other elements into unusually high oxidation numbers, as in AgF2, PtF6, and IF7.
Fluorine is so reactive that it is difficult to find a container in which it can be stored. F2 attacks both glass and quartz, for example, and causes most metals to burst into flame. Fluorine is handled in equipment built out of certain alloys of copper and nickel. It still reacts with these alloys, but it forms a layer of a fluoride on the surface that protects the metal from further reaction.
Fluorine is used in the manufacture of Teflon or poly(tetrafluoroethylene), (C2F4)n which is used for everything from linings for pots and pans to gaskets that are inert to chemical reactions. Large amounts of fluorine are also consumed each year to make the freons (such as CCl2F2) used in refrigerators.
Chlorine (Cl2) is a highly toxic gas with a pale yellow-green color. Chlorine is a very strong oxidizing agent, which is used commercially as a bleaching agent and as a disinfectant. It is strong enough to oxidize the dyes that give wood pulp its yellow or brown color, for example, thereby bleaching out this color, and strong enough to destroy bacteria and thereby act as a germicide. Large quantities of chlorine are used each year to make solvents such as carbon tetrachloride (CCl4), chloroform (CHCl3), dichloroethylene (C2H2Cl2), and trichloroethylene (C2HCl3).
Bromine (Br2) is a reddish-orange liquid with an unpleasant, choking odor. The name of the element, in fact, comes from the Greek stem bromos, “stench.” Bromine is used to prepare flame retardants, fire-extinguishing agents, sedatives, antiknock agents for gasoline, and insecticides.
Iodine is an intensely colored solid with an almost metallic luster. This solid is relatively volatile, and it sublimes when heated to form a violet-colored gas. Iodine has been used for many years as a disinfectant in “tincture of iodine.” Iodine compounds are used as catalysts, drugs, and dyes. Silver iodide (AgI) plays an important role in the photographic process and in attempts to make rain by seeding clouds. Iodide is also added to salt to protect against goiter, an iodine deficiency disease characterized by a swelling of the thyroid gland.
Some of the chemical and physical properties of the halogens are summarized in the table below. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the melting point, boiling point, intensity of the color of the halogen, the radius of the corresponding halide ion, and the density of the element. On the other hand, there is a regular decrease in the first ionization energy as we go down this column. As a result, there is a regular decrease in the oxidizing strength of the halogens from fluorine to iodine.
| F2> Cl2> Br2> I2 |
| oxidizing strength |
This trend is mirrored by an increase in the reducing strength of the corresponding halides.
| I–> Br–> Cl–> F– |
| reducing strength |
Some Properties of F2, Cl2, Br2, and I2
| Melting Point (C) | Boiling Point (C) | Color | Natural Abundance (ppm) | 1st Ionization Energy (kJ/mol) | Electron Affinity (kJ/mol) | Ionic Radius (nm) | Density (g/cm3) | |||||||||
| F2 | -218.6 | -188.1 | colorless | 544 | 1680.6 | 322.6 | 0.133 | 1.513 | ||||||||
| Cl2 | -101.0 | -34.0 | pale green | 126 | 1255.7 | 348.5 | 0.184 | 1.655 | ||||||||
| Br2 | -7.3 | 59.5 | dark red-brown | 2.5 | 1142.7 | 324.7 | 0.196 | 3.187 | ||||||||
| I2 | 113.6 | 185.2 | very dark violet almost black | 0.46 | 1008.7 | 295.5 | 0.220 | 3.960 |
Methods of Preparing the Halogens from their Halides
The halogens can be made by reacting a solution of the halide ion with any substance that is a stronger oxidizing agent. Iodine, for example, can be made by reacting the iodide ion with either bromine or chlorine.
| 2 I–(aq) | + | Br2(aq) | I2(aq) | + | 2 Br–(aq) |
Bromine was first prepared by A. J. Balard in 1826 by reacting bromide ions with a solution of Cl2 dissolved in water.
| 2 Br–(aq) | + | Cl2(aq) | Br2(aq) | + | 2 Cl–(aq) |
To prepare Cl2, we need a particularly strong oxidizing agent, such as manganese dioxide (MnO2).
| 2 Cl–(aq) | + | MnO2(aq) | + | 4 H+(aq) | Cl2(aq) | + | Mn2+(aq) | + | 2 H2O(l) |
The synthesis of fluorine escaped the efforts of chemists for almost 100 years. Part of the problem was finding an oxidizing agent strong enough to oxidize the F– ion to F2. The task of preparing fluorine was made even more difficult by the extraordinary toxicity of both F2 and the hydrogen fluoride (HF) used to make it.
The best way of producing a strong reducing agent is to pass an electric current through a salt of the metal. Sodium, for example, can be prepared by the electrolysis of molten sodium chloride.
| electrolysis | ||||
| 2 NaCl(l) | 2 Na(s) | + | Cl2(g) |
In theory, the same process can be used to generate strong oxidizing agents, such as F2.
Attempts to prepare fluorine by electrolysis, however, were initially unsuccessful. Humphry Davy, who prepared potassium, sodium, barium, strontium, calcium, and magnesium by electrolysis repeatedly tried to prepare F2 by the electrolysis of fluorite (CaF2), and succeeded only in ruining his health. Joseph Louis Gay-Lussac and Louis Jacques Thenard, who prepared elemental boron for the first time, also tried to prepare fluorine and suffered from very painful exposures to hydrogen fluoride. George and Thomas Knox were badly poisoned during their attempts to make fluorine, and both Paulin Louyet and Jerome Nickles died from fluorine poisoning.
Finally, in 1886 Henri Moissan successfully isolated F2 gas from the electrolysis of a mixed salt of KF and HF and noted that crystals of silicon burst into flame when mixed with this gas. Electrolysis of KHF2 is still used to prepare fluorine today, as shown in the figure below.
| electrolysis | ||||||
| 2 KHF2(s) | H2(g) | + | F2(g) | + | 2 KF(s) |
Common Oxidation Numbers for the Halogens
Fluorine is the most electronegative element in the periodic table. As a result, it has an oxidation number of -1 in all its compounds. Because chlorine, bromine, and iodine are less electronegative, it is possible to prepare compounds in which these elements have oxidation numbers of +1, +3, +5, and +7, as shown in the table below.
Common Oxidation Numbers for the Halogens
| Oxidation Number | Examples | |
| -1 | CaF2, HCl, NaBr, AgI | |
| 0 | F2, Cl2, Br2, I2 | |
| +1 | HClO, ClF | |
| +3 | HClO2, ClF3 | |
| +5 | HClO3, BrF5, BrF6–, IF5 | |
| +7 | HClO4, BrF6+, IF7 |
General Trends in Halogen Chemistry
There are several patterns in the chemistry of the halogens.
The Hydrogen Halides (HX)
The hydrogen halides are compounds that contain hydrogen attached to one of the halogens (HF, HCl, HBr, and HI). These compounds are all colorless gases, which are soluble in water. Up to 512 mL of HCl gas can dissolve in a single mL of water at 0oC and 1 atm, for example. Each of the hydrogen halides ionizes to at least some extent when it dissolves in water.
| H2O | ||||
| HCl(g) | H+(aq) | + | Cl–(aq) |
Several of the hydrogen halides can be prepared directly from the elements. Mixtures of H2 and Cl2, for example, react with explosive violence in the presence of light to form HCl.
| H2(g) | + | Cl2(g) | 2 HCl(g) |
Because chemists are usually more interested in aqueous solutions of these compounds than the pure gases, these compounds are usually synthesized in water. Aqueous solutions of the hydrogen halides are often called mineral acids because they are literally acids prepared from minerals. Hydrochloric acid is prepared by reacting table salt with sulfuric acid, for example, and hydrofluoric acid is prepared from fluorite and sulfuric acid.
| 2 NaCl(s) | + | H2SO4(aq) | 2 HCl(aq) | + | Na2SO4(aq) | |
| CaF2(s) | + | H2SO4(aq) | 2 HF(aq) | + | CaSO4(aq) |
These acids are purified by taking advantage of the ease with which HF and HCl gas boil out of these solutions. The gas given off when one of these solutions is heated is collected and then redissolved in water to give relatively pure samples of the mineral acid.
Any of the methods listed below, can be used to prepare chlorine in the laboratory.
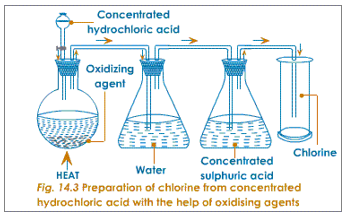
Chlorine can be prepared by removing the hydrogen from hydrochloric acid using an oxidizing agent. Any oxidising agent such as manganese dioxide, lead dioxide, trilead tetroxide, potassium permanganate or potassium dichromate can be used. Firstly, the oxidising agents are taken in the round bottomed flask. Concentrated hydrochloric acid is then added through a thistle funnel. This mixture is then heated. The oxygen of the oxidizing agents combines with the hydrogen of the hydrochloric acid leaving behind chlorine i.e. hydrogen is removed from hydrochloric acid. The metallic ions of the oxidising agents combine with part of chlorine to form the respective chlorides.
Remember:-
No heating is required in when potassium permanganate is used as an oxidizing agent in the above method of preparing chlorine.
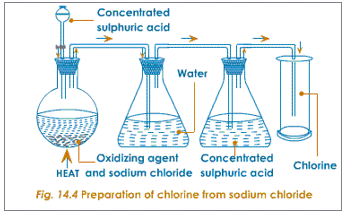
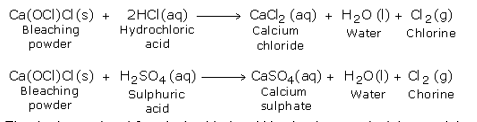
Chlorine can be prepared by the action of hot concentrated sulphuric acid on a mixture of a chloride salt and an oxidising agent. Sodium chloride, being the cheapest and the most easily available chloride salt, is used along with manganese dioxide as the oxidising agent.
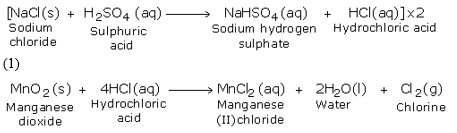
A mixture of almost equal quantity of sodium chloride and manganese dioxide is taken in a round bottomed flask. Concentrated sulphuric acid is then poured through the thistle funnel. The reaction takes place in two stages. In the first stage sulphuric acid reacts with the chloride to form hydrochloric acid. In the second stage the hydrochloric acid so formed combines with the oxidising agent to liberate chlorine.
(2) Manganese (II) chloride, formed during the reaction, reacts with sulphuric acid to form manganese (II) sulphate and hydrochloric acid as under:
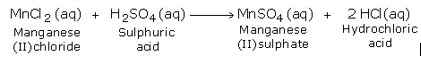
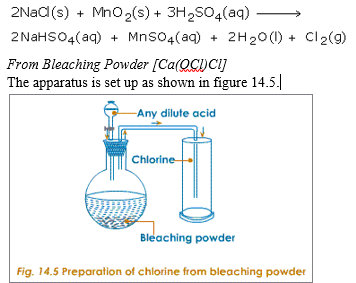
Bleaching powder is taken in a round bottom flask. Any dilute mineral acid is poured through the thistle funnel. Chlorine can be prepared by dropping any acid on bleaching powder.
The chorine produced from hydrochloric acid by the above method, is passed through two wash bottles. The first wash bottle contains water, to remove traces of hydrogen chloride gas from chlorine. The second wash bottle contains concentrated sulphuric acid to dry the gas.
Chlorine is mostly obtained as a by-product during the manufacture of caustic soda, by the electrolysis of brine or molten sodium chloride. Hence chlorine is rather prepared by cheap methods. During this electrolysis, chlorine is liberated at the anode.
Remember:-
As chlorine is denser than air it is collected by the upward displacement of air.
The Interhalogen Compounds
Interhalogen compounds are formed by reactions between different halogens. All possible interhalogen compounds of the type XY are known. Bromine reacts with chlorine, for example, to give BrCl, which is a gas at room temperature.
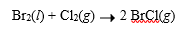
Interhalogen compounds with the general formulas XY3, XY5, and even XY7 are formed when pairs of halogens react. Chlorine reacts with fluorine, for example, to form chlorine trifluoride.
| Cl2(g) | + | 3 F2(g) | 2 ClF3(g) |
These compounds are easiest to form when Y is fluorine. Iodine is the only halogen that forms an XY7interhalogen compound, and it does so only with fluorine.
ClF3 and BrF5 are extremely reactive compounds. ClF3 is so reactive that wood, asbestos, and even water spontaneously burn in its presence. These compounds are excellent fluorinating agents, which tend to react with each other to form positive ions such as ClF2+ and BrF4+ and negative ions such as IF2– and BrF6–.
| 2 BrF5(l) | [BrF4+][BrF6–](s) |
Neutral Oxides of the Halogens
Under certain conditions, it is possible to isolate neutral oxides of the halogens, such as Cl2O, Cl2O3, ClO2, Cl2O4, Cl2O6, and Cl2O7. Cl2O7, for example, can be obtained by dehydrating perchloric acid, HClO4. These oxides are notoriously unstable compounds that explode when subjected to either thermal or physical shock. Some are so unstable they detonate when warmed to temperatures above -40oC.
Oxyacids of the Halogens and Their Salts
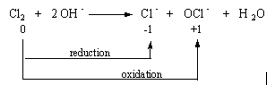
Chlorine reacts with the OH– ion to form chloride ions and hypochlorite (OCl–) ions.
| Cl2(aq) | + | 2 OH–(aq) | Cl–(aq) | + | OCl–(aq) | + | H2O(l) |
This is a disproportionation reaction in which one-half of the chlorine atoms are oxidized to hypochlorite ions and the other half are reduced to chloride ions.
When the solution is hot, this reaction gives a mixture of the chloride and chlorate (ClO3–) ions.
| 3 Cl2(aq) | + | 6 OH–(aq) | 5 Cl–(aq) | + | ClO3–(aq) | + | 3 H2O(l) |
Under carefully controlled conditions, it is possible to convert a mixture of the chlorate and hypochlorite ions into a solution that contains the chlorite (ClO2–) ion.
| ClO3–(aq) | + | ClO–(aq) | 2 ClO2– (aq) |
The last member of this class of compounds, the perchlorate ion (ClO4–), is made by electrolyzing solutions of the chlorate ion.
The names of the oxyanions of the halogens use the endings –ite and –ate to indicate low and high oxidation numbers and the prefixes hypo– and per– to indicate the very lowest and very highest oxidation numbers, as shown in the table below. Each of these ions can be converted into an oxyacid, which is named by replacing the –ite ending with –ous and the –ate ending with –ic.
Oxyanions and Oxyacids of Chlorine
| Oxyanions | Oxyacids | ||||||||
| Oxidation State of the Chlorine | Compound | Name | Compound | Name | |||||
| +1 | ClO– | hypochlorite | HClO | hypochlorous acid | |||||
| +3 | ClO2– | chlorite | HOClO | chlorous acid | |||||
| +5 | ClO3– | chlorate | HOClO2 | chloric acid | |||||
| +7 | ClO4– | perchlorate | HOClO3 | perchloric acid | |||||
EVALUATION
1.Arrange Flourine ,Chlorine,Bromine and Iodine inorder of increasing oxidizing ability
2.Why is the preparation of Halogens carried out in the Fume cupboard?
3.Itemize using relevant chemical equation where necessary how you would test for chlorine in the laboratory.
4.Explain what happen when chlorine water is exposed to sunlight.
post your answers on the forum for review
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 