Nitrogen has 5 electrons in its valence shell. It has a valency of 3 with respect to hydrogen and a valency up to 5 with respect to oxygen. So, it can combine with various elements to form many compounds.
The well known compounds of nitrogen are
- Hydrazine
- Hydrazoic acid
- Hydroxylamine
- Nitrous oxide
- Nitric oxide
- Di nitrogen trioxide
- Nitrogen dioxide
Hydrazine
- This compound can be regarded as being formed by replacing one H atom of NH3 molecule by NH2 group. This was first prepared by Curtius in 1889.
- Free hydrazine is a colorless liquid, very hygroscopic and soluble in alcohol and water. It is not very stable.
- On exposure to air, it readily absorbs carbon dioxide and moisture.
- It dissolves sulfur, arsenic, selenium and phosphorus. Its boiling point is 113.5°C and melting point is 1.4°C.
Hydrazoic acid
- This is also called as azoemide.
- The formula of hydrazoic acid is N3H.
- This is a colorless volatile liquid. Its boiling point is 37°C.
- It is extremely poisonous and dissociates into N2 and H2 with the evolution of a large amount of heat.
Hydroxylamine
- It is a hydroxy derivative of NH3 and was discovered by Lossen in 1865.
- As a rule only the salts of hydroxylamine are prepared because the preparation of free hydroxylamine is difficult and not without hazards under some conditions.
- It is a needle-like white substance.
- Melting point is 33°C and boiling point is 58°C at 22 mm pressure.
- It decomposes at steam temperature.
- It is readily soluble in water but not much in alcohol.
- It can be crystallized out from its ethereal solution.
- Its solution in water is a weak base
- It explodes with halogen and also with permanganate and dichromate.
- It is very unstable and the solid decomposes slowly above 15°C.
Nitric Oxide
- This oxide is also called as nitrous oxide and is produced when a mixture of nitrogen and oxygen is passed through an electric ar
- It is a colorless gas, heavier than air and very sparingly soluble in water.
- Under pressure, it changes to a colorless liquid under -151°C.
- The gas is neutral to litmus solution.
- It combines with O2 to form brown fumes and nitrogen peroxide(NO2).
- It reacts with burning charcoal, S, P to give oxides.
Nitrous Oxide
- The formula of this oxide is N2O.
- When this oxide is inhaled in small quantities, it produces hysterical laughter and for this reason this oxide is also called as laughing gas.
- It is prepared by heating a mixture of NaNO3 and (NH4)2SO4
- Nitrous oxide is a colorless gas having a faint, sweetish smell and a sweet taste.
- When inhaled for a long time, it produces insensibility.
- Large quantities may prove fatal.
- This is soluble in water and the solution has a sweetish taste.
- That is why it is collected over hot water.
- It is heavier than air. It can easily be liquified at 0°C and at a pressure of 30 atm.
- The liquid boils at -89.5°C.
- The gas is neutral to litmus.
Di nitrogen Trioxide
- The formula is N2O3.
- This oxide is also called nitrogen sesquioxide.
- It is also called nitrous anhydride. This means it is the anhydride of HNO2, since it gives HNO2 when heated with H2O.
- Nitrogen trioxide is prepared by the reduction of nitric acid with arseniousoxid
- It is a red colored gas.
- On condensing, it gives a dark blue liquid.
- It is acidic in nature and reacts with sodium hydroxide to form sodium nitrite
The Synthesis of ammonia – The Haber Process of Nitrogen Fixation
- Ammonia gas is synthesised in the chemical industry by reacting nitrogen gas with hydrogen gas in what is known as the Haber-Bosch Process, named after two highly inventive and subsequently famous chemists.
- The Haber synthesis of ammonia is important for agriculture because nitrogen is an important element for plant growth.
- But, it is a very stable molecule and only a few plants like legumes (peas, beans etc.) can directly ‘fix’ nitrogen the from air and incorporate it into protein molecules.
- The Haber synthesis allows the efficient mass production of ‘artificial fertilisers’.
- If the chemical feedstocks for the Haber Process are nitrogen and hydrogen, where do we get these materials from?
- The nitrogen was once obtained from the fractional distillation of liquified air (80% N2).
- the air is filtered to remove dust and then compressed under high pressure.
- The filtered air is cooled and water condensed out, and then carbon dioxide freezes out at -78oC.
- The air further cooled to form liquid air (liquefaction) at around -200oC.
- The liquid air is fractionally distilled at low temperature to separate oxygen (used in welding, hospitals etc.), nitrogen (for making ammonia), Noble Gases e.g. argon for light bulbs, helium for balloons).
- Oxygen and argon are very close in boiling point and initially come out in the same fraction so a further fractional distillation is needed to separate them.
- However, nitrogen is also produced by ‘deoxygenating’ air by combustion with methane.
- The hydrogen is made by (i) reacting methane (natural gas) and water or (ii) from cracking hydrocarbons (both reactions are done at high temperature with a catalyst).
- (i) methane + water (steam) ==> hydrogen + carbon monoxide
- CH4 + H2O ==> 3H2 + CO
- The ‘deadly’ carbon monoxide can be reacted with water in a 2nd stage to make more hydrogen.
- CO + H2O ==> CO2 + H2
- and the carbon dioxide is removed to give the desired hydrogen gas.
- These are called ‘reforming’ reactions.
- CO + H2O ==> CO2 + H2
- or (ii) from cracking an alkane hydrocarbon from crude oil
- e.g. C8H18==> C8H16 + H2
- AND, but just in passing and convenient ….
- Other uses of hydrogen
- Hydrogen-oxygen fuel cells to make electricity on small-scale.
- Hydrogenation vegetable oils to make margarine.
- Reducing metal oxides to free the metal.
- An atomic hydrogen-oxygen welding torch.
- Inflating weather balloons.
- (i) methane + water (steam) ==> hydrogen + carbon monoxide
- The balanced equation for the Haber Synthesis reversible reaction is …
- N2(g) + 3H2(g) 2NH3(g) (plus 92 kJ of heat energy given out, exothermic reaction)
- .. which means an equilibrium will form, so there is no chance of 100% yield even if you use, as you actually do, the theoretical reactant ratio of nitrogen : hydrogen of 1 : 3 !
- In forming ammonia 92kJ of heat energy is given out (i.e. exothermic, 46kJ of heat released per mole of ammonia formed).
- Also, four moles of ‘reactant’ gas form two moles of ‘product’ gas, so there is a net decrease in gas molecules on forming ammonia.
- So applying the equilibrium rules, the formation of ammonia should be favoured by …
- (a) Using high pressure because you are going from 4 to 2 gas molecules, so high pressure favours the forward reaction to give fewer gas molecules.
- The high pressure also speeds up the reaction because it effectively increases the concentration of the gas molecules,
- but, the higher pressure means more dangerous and more costly engineering, so a compromise needed.
- Does this prediction match the graph?
- (b) Carrying out the reaction at a low temperature, favouring the forward reaction,
- because it is an exothermic reaction favoured by lowering the temperature,
- but, this may produce too slow a rate of reaction, so a compromise needed.
- Does this prediction match the graph?
- Therefore, the idea is to use a set of optimum conditions to get the most efficient yield of ammonia and this involves getting a low % yield (e.g. 8% – 15% conversion) but fast.
- Described below are the conditions to give the most economic production of ammonia.
- These arguments make the point that the yield of an equilibrium reaction depends on the conditions used.
- The word ‘yield‘ means how much product you get compared to the theoretical maximum possible if the forward reaction goes 100% that way.
- .
- (a) Using high pressure because you are going from 4 to 2 gas molecules, so high pressure favours the forward reaction to give fewer gas molecules.
- In industry pressures of 200 – 300 times normal atmospheric pressure are used in line with the theory (200-300 atm).
- Theoretically a low temperature would give a high yield of ammonia BUT …
- Nitrogen is very stable molecule and not very reactive i.e. chemically inert, so the rate of reaction is too slow at low temperatures.
- To speed up the reaction an iron catalyst is used as well as a higher temperature (e.g. 400-450oC).
- The higher temperature is an economic compromise, i.e. it is more economic to get a low yield fast, than a high yield slowly!
- Note: a catalyst does NOT affect the yield of a reaction, i.e. the equilibrium position BUT you do get there faster!
- Note: a catalyst does NOT affect the yield of a reaction, i.e. the equilibrium position BUT you do get there faster!
The initial yield is 6%-8%, but unreacted gases are recycled to raise this to nearer 100%
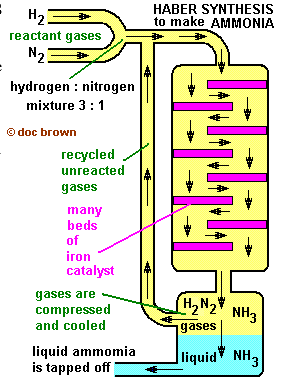
- eventually … read on …
- To sum up: A low % yield of ammonia is produced quickly at moderately high temperatures and pressure in the presence of an iron catalyst, and is more economic than getting a higher % equilibrium yield of ammonia at a more costly high pressure and a slower lower temperature reaction.
- Using an effective iron catalyst can the reduce the cost of manufacturing ammonia by increasing the rate of reaction (more efficient) and lowering the energy requirements if the process can be done at lower temperatures (activation energy reduced).
- Increasing the rate of reaction saves time and operating at a lower temperature saves energy and therefore saves money.
- However, catalysts can be very specialised and expensive to produce and they get contaminated (‘poisoned’) and become less efficient, in this case sulfur compounds contaminate the iron catalyst.
- So the iron catalyst might have to be extracted and cleaned up, but if a true catalyst (and it is), this’ refurbishment’ should enable the iron catalyst to be reused.
- Remember, theoretically catalysts take part in the reaction, but are not consumed in the reaction and can be reused over and over again.
The summary of the formation of ammonia
nitrogen + hydrogen ammonia
N2(g) + 3H2(g) 2NH3(g) (plus 92 kJ heat energy, exothermic)
Ammonia formation is favouredby …
Rule 1. Lowering the temperature, because it is an exothermic reaction, but this may make it too slow, compromise required.
Rule 2. Increasing pressure, because there is a reduction in the molecules of gas, but the higher the pressure, the more costly the engineering.
Rule 4. An iron catalyst speeds up the reaction, but has no effect on the % ammonia in the reacted mixture exiting the reactor chamber in the chemical plant of a Haber synthesis process.
The Uses of Ammonia
Ammonia is used to make ammonium salts (mostly artificial fertilisers) and is used in the chemical industry in the manufacture of explosives, nitric acid and nitrates, pharmaceutical products and plastics.
(a) Ammonia is used to manufacture nitric acid
- Ammonia is oxidised with oxygen from air using a hot platinum catalyst to form nitrogen monoxide and water.
- 4NH3(g) + 5O2(g)==> 4NO(g) + 6H2O(g)
- The gas is cooled and reacted with more oxygen to form nitrogen dioxide.
- 2NO(g) + O2(g) ==>2NO2(g)
- This is reacted with more oxygen and water to form nitric acid.
- 4NO2(g)+ O2(g) + 2H2O(l)==> 4HNO3(aq)
- Nitric acid is used to make nitro-aromatic compounds from which dyes are made.
- It is also used in the manufacture of artificial nitrogenous fertilisers (like ammonium nitrate, see below).
(b) Ammonia is used to manufacture ‘artificial’ nitrogenous fertilisers
- Ammonia is a pungent smelling alkaline gas that is very soluble in water.
- The gas or solution turns litmus or universal indicator blue because it is a soluble weak base or weak alkali and is neutralised by acids to form salts.
- Ammonia is a synthetic rich source of artificial nitrogenous fertilisers essential for increased growth of plants e.g. cereal crops.
- Ammonium salts are used as ‘artificial’ or ‘synthesised’ fertilisers i.e. nitrogenous fertilisers ‘man-made’ in a chemical works, and used as an alternative to natural manure or compost etc.
- Ammonia is a base, and fertiliser salts are made by neutralising ammonia solution with the appropriate acid.
- The resulting solution is heated, evaporating the water to crystallise the salt e.g. with correct equations, with and without state symbols …
(i) ammonia + hydrochloric acid ==> ammonium chloride
NH3 + HCl ==> NH4Cl
NH3(aq) + HCl(aq) ==> NH4Cl(aq)
(ii) ammonia + sulphuric acid ==> ammonium sulphate
2NH3 + H2SO4 ==> (NH4)2SO4
2NH3(aq) + H2SO4(aq)==> (NH4)2SO4(aq)
(iii) ammonia + nitric acid ==> ammonium nitrate
NH3 + HNO3 ==> NH4NO3
NH3(aq) + HNO3(aq)==> NH4NO3(aq)
Reactions (ii) and (iii) are used in fertiliser production, as is reaction (iv)
ammonia + phosphoric acid ==> ammonium phosphate
EVALUATION
1.Describe the following oxides of Nitrogen.
N2O4, NO2 , N2O5 , NO
2.Which of the oxides of nitrogen is a neutral oxide?
a.N2O4 b.NO2 C.N2O5 d.NO
3.Describe the Haber process
4.How is trioxonitrate v acid produced from ammonia?
5.Describe the brown ring experiment.
post your answer on the forum for review
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 