Sulfur belongs to the chalcogen family. Other members of the family are oxygen, selenium, tellurium, and polonium. These elements make up Group 16 (VIA) of the periodic table. The periodic table is a chart that shows how chemical elements are related to each other.
The term chalcogen comes from two Greek words meaning “ore forming.” An ore is a naturally occurring mineral used as a source for an element. Many ores are compounds of a metal and oxygen or a metal and sulfur. Compounds that contain two elements, one of which is sulfur, are called sulfides. For example, a beautiful gold-colored mineral is called pyrite, or “fool’s gold,” because it looks so much like real gold. Pyrite is iron sulfide (FeS2 ).
Sulfur was known to ancient peoples. Its physical and chemical properties are very distinctive. It often occurs as a brilliant yellow powder. When it burns, it produces a clear blue flame and a very strong odor.
SYMBOL
S
ATOMIC NUMBER
16
ATOMIC MASS
32.064
FAMILY
Group 16 (VIA)
Chalcogen
PRONUNCIATION
SUL-fur
Sulfur, also spelled as sulphur, is a very important element in today’s world. Its most important use is in the manufacture of sulfuric acid (H 2 SO 4 ). There is more sulfuric acid made than any other chemical in the world. It has an enormous number of important uses.
Discovery and naming
Sulfur must have been well known to ancient peoples. They sometimes referred to it as brimstone. Sulfur sometimes occurs in bright yellow layers on the top of the earth. It has a sharp, offensive odor. When it burns, it gives off a strong, suffocating smell. The odor is like that produced when a match is struck.
The Bible mentions brimstone in a number of places. For example, Sodom and Gomorrah were two towns destroyed by God for the wicked ways of their citizens: “The Lord rained upon Sodom and upon Gomorrah brimstone and fire.”
But ancient people certainly did not think about sulfur the way modern chemists do. In fact, they used the word “element” to talk about anything that was basic. Ancient Greek philosophers, for example, thought that everything consisted of four elements: earth, fire, water, and air. Other philosophers thought there were only two elements: sulfur and mercury.
But early thinkers were often confused as to what they meant by the word “sulfur.” They often were talking about anything that burned and gave off large amounts of smoke. To them, “sulfur” was really a “burning substance.” It took centuries for scientists to identify sulfur as an element.
Physical properties
Sulfur exists in two allotropic forms. Allotropes are forms of an element with different physical and chemical properties. The two forms of sulfur are known as α-form and β-form (the Greek letters alpha and beta, respectively). Both allotropes are yellow, with the α-form a brighter yellow and the β-form a paler, whitish-yellow. The α-form changes to the β-form at about 94.5°C (202°F). The α-form can be melted at 112.8°C (235.0°F) if it is heated quickly. The β-form has a melting point of 119°C (246°F). The boiling point of the α-form is 444.6°C (832.3°F).
The two allotropes have densities of 2.06 grams per cubic centimeter (α-form) and 1.96 grams per cubic centimeter (β-form). Neither allotrope will dissolve in water. Both are soluble in other Liquids, such as benzene (C 6 H 6 ), carbon tetrachloride (CCl4 ), and carbon disulfide (CS 2 ).
Another allotrope of sulfur is formed when the element is melted. This allotrope has no crystalline shape. It looks like a dark brown, thick, melted plastic.
Chemical properties
Sulfur’s most prominent chemical property is that it burns. When it does so, it gives off a pale blue flame and sulfur dioxide (SO 2 ) gas. Sulfur dioxide has a very obvious strong, choking odor.
Sulfur sometimes occurs in bright yellow layers on the top of the earth. It has a sharp, offensive odor.
Sulfur also combines with most other elements. Sometimes it combines with them easily at room temperature. In other cases, it must be heated. The reaction between magnesium and sulfur is typical. When the two elements are heated, they combine to form magnesium sulfide (MgS):
Sulfur also combines with hydrogen gas:
The compound formed in this reaction is hydrogen sulfide (H 2 S). Hydrogen sulfide has one of the best known odors of all compounds. It smells like rotten eggs. Hydrogen sulfide is added to natural gas (methane) used in homes for cooking and heating. Methane is odorless. So the unique smell of hydrogen sulfide makes it easy to know when there is a methane leak.
Occurrence in nature
At one time, sulfur occurred in layers along the Earth’s surface. They were easy for humans to find and take. Deposits like these are more difficult to find today. One place they still occur is in the vicinity of volcanoes. Sulfur is released from volcanoes as a gas. When it reaches the cold air, it changes back to a solid. It forms beautiful yellow deposits along the edge of a volcano.
Large supplies of sulfur still occur underground. They are removed by the Frasch process (see accompanying sidebar).
Sulfur also occurs in a number of important minerals. Some examples are barite, or barium sulfate (BaSO4 ); celestite, or strontium sulfate (SrSO4 ); cinnabar, or mercury sulfide (HgS); galena, or lead sulfide (PbS); pyrites, or iron sulfide (FeS2 ); sphalerite, or zinc sulfide (ZnS); and stibnite, or antimony sulfide (Sb 2 S 3 ).
The abundance of sulfur in the Earth’s crust is thought to be about 0.05 percent. It ranks about number 16 among the elements in terms of their abundance in the earth. It is more abundant than carbon, but less abundant than barium or strontium.
The largest producers of sulfur in the world are the United States, Canada, China, Russia, Mexico, and Japan. In 1996, the United States produced about 11,800,000 metric tons of sulfur. It is mined in 30 states, Puerto Rico, and the U.S. Virgin Islands.
Isotopes
There are four naturally occurring isotopes of sulfur: sulfur-32, sulfur-33, sulfur-34, and sulfur-36. Isotopes are two or more forms of an element. Isotopes differ from each other according to their mass number. The number written to the right of the element’s name is the mass number. The mass number represents the number of protons plus neutrons in the nucleus of an atom of the element. The number of protons determines the element, but the number of neutrons in the atom of any one element can vary. Each variation is an isotope.
Sulfur occurs in the vicinity of volcanoes.
Six radioactive isotopes of sulfur are known also. A radioactive isotope is one that breaks apart and gives off some form of radiation. Radioactive isotopes are produced when very small particles are fired at atoms. These particles stick in the atoms and make them radioactive.
One radioactive isotope of sulfur, sulfur-35, is used commercially. In medicine, the isotope is used to study the way fluids occur inside the body. It also has applications in research as a tracer. A tracer is a radioactive isotope whose presence in a system can easily be detected. The isotope is injected into the system at some point. Inside the system, the isotope gives off radiation. That radiation can be followed by means of detectors placed around the system.
As an example, a company that makes rubber tires might want to know what happens to the sulfur added to tires. Sulfur-35 is added to rubber along with non-radioactive sulfur. Researchers follow the radioactive isotope in the tires to see what happens to the sulfur when the tires are used.
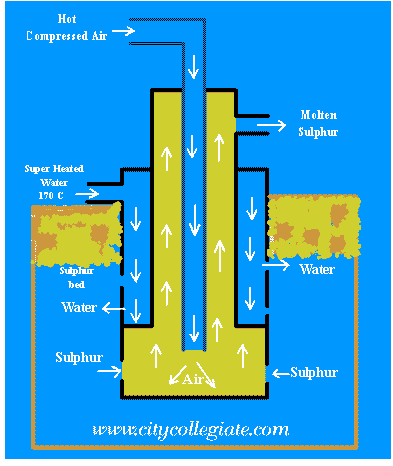
The Frasch method of removing sulfur
T heFrasch method is one of the most famous mining systems ever invented. It was developed by German-American chemist Herman Frasch (1851-1914) in 1887.
The Frasch method is based on the low melting point of sulfur. The element melts at a temperature slightly higher than that of boiling water (100°C). Here is how the method works:
A set of three nested pipes (one inside each other) is sunk into the ground. The innermost pipe has a diameter of about an inch. The middle pipe has a diameter of about four inches. And the outer pipe has a diameter of about eight inches.
A stream of superheated water is injected into the outer pipe. Superheated water is water that is hotter than its boiling point, but that has not started to boil. Superheated water can be made by raising the pressure on the water. Its temperature can reach 160°C (320°F).
The superheated water passes down the outer pipe into the underground sulfur, causing it to melt. The molten (melted) sulfur forms a lake at the bottom of the pipe.
At the same time, a stream of hot air under pressure is forced down the innermost (one-inch) pipe. The hot air stirs up the molten sulfur and hot water at the bottom of the pipe. A foamy, soupy mixture of sulfur and water is formed. The mixture is forced upward through the middle pipe. When it reaches the surface, it is collected. The sulfur cools and separates from the water.
Similar applications of sulfur-35 involve studying sulfur in steel when it is made, seeing how sulfur affects the way engines operate, following what happens when proteins (which contain sulfur) are digested, and learning how drugs that contain sulfur are processed in the body.
| METHOD | ||
| Superheated steam at 170oC under 16 atmp is pumped down the outer most pipe . Since melting point of sulphur is 113oC , it is melted and collects at the bottom. Hot compressed air is blown down the inner most pipe to produce foam of molten sulphur . This foam rises through the annular space between the inner most pipe and the next. The foam of sulphur is collected in wooden tubs. After few hours, sulphur is converted into solid blocks. | ||
EVALUATION
1.Sulphur iv oxide bleaches by a.oxidation b.reduction c.decomposition d.carboxylation
2.sulphur iv oxide is used for these except a.germicide and fungicide b.refrigerant c.preszerving liquids like orange juice d.used for restoring ozone layer.
3.sulphur reacts with soft rubber used to harden it is a. direct linkage b.polymerization c.crosss linkage d.smoking
4.The bleaching property of SO2 is due to
a.Acidic nature
b.basic nature
c.reducing property
d.oxidizing property
5. What is the transition temperature for the two allotropes of sulphur?
a.250C b.780C c.960C d.100 0C
Describe the following under Sulphur.
Physical properties, chemical properties , occurrence ,Extraction
Post your answers on the forum for review
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 