Reversible reactions
A reversible reaction is one which can be made to go in either direction depending on the conditions.
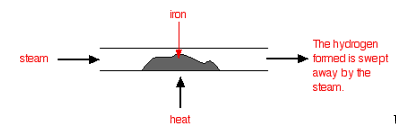
If you pass steam over hot iron the steam reacts with the iron to produce a black, magnetic oxide of iron called triiron tetroxide, Fe3O4.
The hydrogen produced in the reaction is swept away by the stream of steam
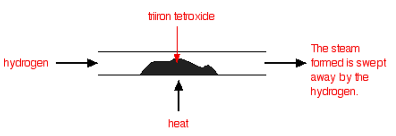
| Under different conditions, the products of this reaction will also react together. Hydrogen passed over hot triiron tetroxide reduces it to iron. Steam is also produced. |

| This time the steam produced in the reaction is swept away by the stream of hydrogen. |
| These reactions are reversible, but under the conditions normally used, they become one-way reactions. The products aren’t left in contact with each other, so the reverse reaction can’t happen.Reversible reactions happening in a closed systemA closed system is one in which no substances are either added to the system or lost from it. Energy can, however, be transferred in or out at will. |
In the example we’ve been looking at, you would have to imagine iron being heated in steam in a closed container. Heat is being added to the system, but none of the substances in the reaction can escape. The system is closed.
As the triiron tetroxide and hydrogen start to be formed, they will also react again to give the original iron and steam. So, if you analysed the mixture after a while, what would you find?
You would find that you had established what is known as a dynamic equilibrium.
A dynamic equilibrium occurs when you have a reversible reaction in a closed system. Nothing can be added to the system or taken away from it apart from energy.
At equilibrium, the quantities of everything present in the mixture remain constant, although the reactions are still continuing. This is because the rates of the forward and the back reactions are equal.
If you change the conditions in a way which changes the relative rates of the forward and back reactions you will change the position of equilibrium – in other words, change the proportions of the various substances present in the equilibrium mixture.
LE CHATELIER’S PRINCIPLE
| Using Le Chatelier’s PrincipleA statement of Le Chatelier’s PrincipleIf a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to counteract the change. Using Le Chatelier’s Principle with a change of concentration |
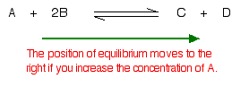
Suppose you have an equilibrium established between four substances A, B, C and D

| What would happen if you changed the conditions by increasing the concentration of A |
| According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the concentration of A decreases again – by reacting it with B and turning it into C + D. The position of equilibrium moves to the right. |
| This is a useful way of converting the maximum possible amount of B into C and D. You might use it if, for example, B was a relatively expensive material whereas A was cheap and plentiful.What would happen if you changed the conditions by decreasing the concentration of A? |
According to Le Chatelier, the position of equilibrium will move so that the concentration of A increases again. That means that more C and D will react to replace the A that has been removed. The position of equilibrium moves to the left

This is esssentially what happens if you remove one of the products of the reaction as soon as it is formed. If, for example, you removed C as soon as it was formed, the position of equilibrium would move to the right to replace it. If you kept on removing it, the equilibrium position would keep on moving rightwards – turning this into a one-way reaction.
Using Le Chatelier’s Principle with a change of pressure
This only applies to reactions involving gases:
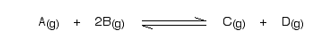
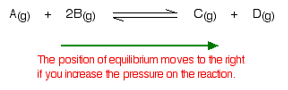
What would happen if you changed the conditions by increasing the pressure?
According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the pressure is reduced again.
Pressure is caused by gas molecules hitting the sides of their container. The more molecules you have in the container, the higher the pressure will be. The system can reduce the pressure by reacting in such a way as to produce fewer molecules.
In this case, there are 3 molecules on the left-hand side of the equation, but only 2 on the right. By forming more C and D, the system causes the pressure to reduce.
Increasing the pressure on a gas reaction shifts the position of equilibrium towards the side with fewer molecules.
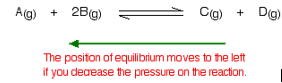
What would happen if you changed the conditions by decreasing the pressure?
The equilibrium will move in such a way that the pressure increases again. It can do that by producing more molecules. In this case, the position of equilibrium will move towards the left-hand side of the reaction.
What happens if there are the same number of molecules on both sides of the equilibrium reaction?
In this case, increasing the pressure has no effect whatsoever on the position of the equilibrium. Because you have the same numbers of molecules on both sides, the equilibrium can’t move in any way that will reduce the pressure again.
Using Le Chatelier’s Principle with a change of temperature
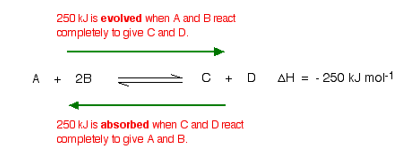
For this, you need to know whether heat is given out or absorbed during the reaction. Assume that our forward reaction is exothermic (heat is evolved):
WHAT CHANGES THE POSITION OF AN EQUILIBRIUM?
By ‘position of an equilibrium’ we mean what are the relative amounts of reactants and products.
AND, importantly, what changes the position of an equilibrium?
In other words, what factors affect the position of an equilibrium?
In a reversible reaction, changing the reaction conditions e.g. concentration, pressure or temperature will change the net direction the reaction goes i.e. more to the right (forward) or more to left (backward) and this must inevitably change the position of the equilibrium.
If you enforce a change on a chemical system at equilibrium, then the system will respond to alter the equilibrium position, BUT the system responds in a way to minimise the enforced change.
The most important factors to consider that strongly influence the position of an equilibrium are temperature, pressure (if gases) and concentration (if solution).
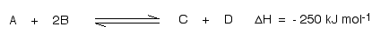
This shows that 250 kJ is evolved (hence the negative sign) when 1 mole of A re acts completely with 2 moles of B. For reversible reactions, the value is always given as if the reaction was one-way in the forward direction.
The back reaction (the conversion of C and D into A and B) would be endothermic by exactly the same amount
What would happen if you changed the conditions by increasing the temperature?
According to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change. That means that the position of equilibrium will move so that the temperature is reduced again.
Suppose the system is in equilibrium at 300°C, and you increase the temperature to 500°C. How can the reaction counteract the change you have made? How can it cool itself down again?
To cool down, it needs to absorb the extra heat that you have just put in. In the case we are looking at, the back reaction absorbs heat. The position of equilibrium therefore moves to the left. The new equilibrium mixture contains more A and B, and less C and D.

If you were aiming to make as much C and D as possible, increasing the temperature on a reversible reaction where the forward reaction is exothermic isn’t a good idea!
What would happen if you changed the conditions by decreasing the temperature?
The equilibrium will move in such a way that the temperature increases again.
Suppose the system is in equilibrium at 500°C and you reduce the temperature to 400°C. The reaction will tend to heat itself up again to return to the original temperature. It can do that by favouring the exothermic reaction.
The position of equilibrium will move to the right. More A and B are converted into C and D at the lower temperature.

- Increasing the temperature of a system in dynamic equilibrium favours the endothermic reaction. The system counteracts the change you have made by absorbing the extra heat.
- Decreasing the temperature of a system in dynamic equilibrium favours the exothermic reaction. The system counteracts the change you have made by producing more heat.
| · Chatelier’s Principle and catalystsAdding a catalyst makes absolutely no difference to the position of equilibrium, and Le Chatelier’s Principle doesn’t apply to them. This is because a catalyst speeds up the forward and back reaction to the same extent. Because adding a catalyst doesn’t affect the relative rates of the two reactions, it can’t affect the position of equilibrium. So why use a catalyst? |
- For a dynamic equilibrium to be set up, the rates of the forward reaction and the back reaction have to become equal. This doesn’t happen instantly. For a very slow reaction, it could take years! A catalyst speeds up the rate at which a reaction reaches dynamic equilibrium.
WHAT CHANGES THE POSITION OF AN EQUILIBRIUM?
By ‘position of an equilibrium’ we mean what are the relative amounts of reactants and products.
AND, importantly, what changes the position of an equilibrium?
In other words, what factors affect the position of an equilibrium?
In a reversible reaction, changing the reaction conditions e.g. concentration, pressure or temperature will change the net direction the reaction goes i.e. more to the right (forward) or more to left (backward) and this must inevitably change the position of the equilibrium.
If you enforce a change on a chemical system at equilibrium, then the system will respond to alter the equilibrium position, BUT the system responds in a way to minimise the enforced change.
The most important factors to consider that strongly influence the position of an equilibrium are temperature, pressure (if gases) and concentration (if solution).
What are the RULES GOVERNING THE POSITION OF A CHEMICAL EQUILIBRIUM?
For industrial processes, it is important to maximise the concentration of the desired products and minimise the ‘leftover’ reactants. A set of rules can be used to predict the best reaction conditions to give the highest possible yield of product.
The three rules outlined below are known as Le Chatelier’s Principle. This essentially states that if a change is imposed on a system, the system will change to minimise the enforced change to re-establish equilibrium.
Le Chatelier’s Principle: Rule 1 The effect of temperature change on the position of an equilibrium
Reminder: If a forward reaction is exothermic, the reverse backward reaction is endothermic and vice versa.
If the temperature of a chemical system at equilibrium is increased then the relative amount of products at equilibrium increases for an endothermic reaction, but the relative amount of products at equilibrium decreases for an exothermic reaction.
If the temperature of a chemical system at equilibrium is decreased: then the relative amount of products at equilibrium decreases for an endothermic reaction, but the relative amount of products at equilibrium increases for an exothermic
reaction.
Rule 1a: If the forward reaction forming the products is endothermic, raising the temperature favours its formation increasing the yield of product (lowering the temperature decreases the yield).
So increasing temperature favours the endothermic direction reaction.
The system attempts to absorb the heat and minimise the increase in temperature.
Rule 1b: If the forward reaction forming the products is exothermic, decreasing the temperature favours its formation (increasing temperature decreases the yield).
So decreasing temperature favours the exothermic direction reaction
The system attempts to release heat to minimise the temperature decrease.
Rule 1 examples
The equilibrium between hydrogen gas, gaseous iodine and gaseous hydrogen iodide.
H2(g) + I2(g) 2HI(g) (plus 10 kJ of heat energy, exothermic L to R)
Increasing temperature favours the endothermic direction, backward reaction, some hydrogen iodide will decompose.
Decreasing temperature favours the exothermic reaction, so more hydrogen and iodine react to form hydrogen iodide.
Le Chatelier’s Principle: Rule 2 The effect of changing pressure on the position of an equilibrium
You can increase/decrease the pressure by decreasing/increasing the volume of the gases OR increasing/decreasing the concentration of gases in the same volume.
For reactions involving gases at equilibrium, an increase in pressure causes the equilibrium position to shift towards the side with the smallest number of gaseous molecules as indicated by the balanced symbol equation for that reaction.
A decrease in pressure of chemical reaction system involving gases, causes the equilibrium position to shift towards the side with the larger number of gaseous molecules as indicated by the balanced symbol equation for that reaction.
A correctly balanced equation is important, because ALL gaseous molecules shown in the equation must be taken into account.
Rule 2a:Increasing the pressure favours the side of the equilibrium with the least number of gaseous molecules as shown by the balanced symbol equation.
So increasing pressure favours the reaction direction to reduce the number of gaseous molecules.
The system is changing to minimise the impact of the increase in pressure by removing some gas molecules.
Rule 2b: Decreasing the pressure favours the side of the equilibrium with the most number of gaseous molecules as shown by the balanced symbol equation.
So decreasing pressure favours the reaction direction to produce the most gaseous molecules.
The system is changing to minimise the impact of the decrease in pressure by increasing the number of gas molecules.
Rule 2 examples
(i) N2(g) + 3H2(g) 2NH3(g)
4 gas molecules ==> 2 gas molecules, so to re-establish a dynamic equilibrium …
Increase in pressures favours the forward reaction to reduce the number of gas molecules, so more ammonia formed.
Decrease in pressure encourages the formation of more gas molecules, so some of the ammonia decomposes into nitrogen and hydrogen.
(ii) N2O4(g) 2NO2(g)
1 gas molecule ==> 2 gas molecules, so to re-establish a dynamic equilibrium …
Increase in pressure favours backward direction to reduce the number of gaseous molecules and give more dinitrogen tetroxide.
Decrease in pressure encourages more gas molecules to form, so the forward reaction gives more nitrogen dioxide.
(iii) N2(g) + O2(g) 2NO(g)
2 gas molecules ==> 2 gas molecules
Change in pressure has no effect on equilibrium position
Rules 1 above, and rule 3, below, apply to any reaction, BUT rule 2 above, ONLY applies to a reaction with one or gaseous reactants or gaseous products.
Increase in pressure does not influence the concentration of substances in a solution or solid mixture because they are too dense to be significantly compressed i.e. no effective change in concentration.
The situation is quite different in gases where is a lot of space between the molecules to compress them closer together.
If a reaction involves gases BUT there are equal numbers of gaseous molecules on each side of the equation, increasing or decreasing pressure has no effect on the position of the equilibrium.
e.g the equilibrium position of the reaction to form hydrogen iodide from hydrogen and iodine
H2(g) + I2(g) 2HI(g)
is unaffected by change in pressure, the are two molecules (or moles) of gas on each side of the equation.
Le Chatelier’s Principle: Rule 3 The effect of changing concentration on the position of an equilibrium
If the concentration of any of the reactants or products is changed, the system cannot any longer be at equilibrium.
The concentrations of all the substances will change until equilibrium is reached again.
If the concentration of a reactant is increased, more products will be formed until equilibrium is reached again.
If the concentration of a product is decreased, more reactants will react until equilibrium is reached again.
Rule 3a: If the concentration of a reactant (on the left) is increased, then some of it must change to the products (on the right) to maintain a balanced equilibrium position.
Rule 3b: If the concentration of a reactant (on the left) is decreased, then some of the products (on the right) must change back to reactants to maintain a balanced equilibrium position.
Rule 3 examples
- e.g. nitrogen + hydrogen ammonia
- or N2(g) + 3H2(g) 2NH3(g)
- If the nitrogen or hydrogen concentration was increased, some of this extra gas would change to ammonia.
- If the nitrogen or hydrogen concentration was decreased, some of ammonia would change back to nitrogen and hydrogen.
- At AS-A2 advanced level things can get more complicated e.g. can you figure out why in terms of concentration to maintain the equilibrium balance? (and if a gcse student, don’t worry if you can’t) …
- So in terms of enforced change ==>system response:
- Increasing nitrogen concentration ==> decreases hydrogen concentration and increases ammonia concentration
- Increasing hydrogen concentration ==> decreases nitrogen concentration and increases ammonia concentration
- Increasing ammonia concentration ==> increases both nitrogen and hydrogen concentrations
- Decreasing ammonia concentration ==> decreases both nitrogen and hydrogen concentration
- Decreasing nitrogen concentration ==> increases hydrogen concentration and decreases ammonia concentration
- Decreasing hydrogen concentration ==> increases nitrogen concentration and decreases ammonia concentration
Le Chatelier’s Principle: Rule 4 The effect of using a catalyst on the position of an equilibrium
A catalyst does NOT affect the position of an equilibrium.
You just get to the equilibrium position here faster!
A catalyst usually speeds up both the forward and reverse reaction but there is no way it can influence the final ‘balanced’ concentrations.
However, the importance of a catalyst lies with economics e.g.
(i) bringing about reactions with high activation energies at lower temperatures and so saving the cost on energy,
(ii) and saving time is saving money, i.e. a catalyst increases the efficiency of the chemical process e.g. the Haber synthesis of ammonia.
Rule 4 examples
Iron catalyst in the synthesis of ammonia.
Vanadium pentoxide catalyst in the Contact Process for manufacturing sulfuric acid.
Both of these chemical processes are faster and made economically more efficient by use of a catalyst, but you don’t get a greater % yield in the final reacted mixture.
Applying the rules 1 to 4 to some chemical processes
(a) The formation of calcium oxide (lime) and carbon dioxide from calcium carbonate (limestone)
CaCO3(s) CaO(s) + CO2(g)
The forward reaction is endothermic, 178kJ of heat energy is absorbed (taken in) for every mole of calcium oxide formed.
One mole of gas is formed in the process, so there is a net increase in the moles of gas in lime formation, since there are no gaseous reactants.
From rule 1: increasing the temperature will increase the yield of calcium oxide or lime, CaO which is endothermically formed.
From rule 2: decreasing the pressure will favour the formation of more gas molecules if possible, so more carbon dioxide formed, and hence more lime.
Lime is made commercially by heating limestone to a high temperature (e.g. 1000oC) in a limekiln that is well ventilated (this reduces the carbon dioxide pressure and so reduces the un-desired backward reaction).
(b) The formation of hydrogen chloride from hydrogen and chlorine
H2(g) + Cl2(g) 2HCl(g)
The forward reaction is very exothermic, 184kJ of heat energy is given out in forming hydrogen bromide according to the above equation (184/2 = 92kJ per mole of HCl formed).
There is no net change in the moles of gas (2 moles reactants 2 moles of product)
From rule 1: decreasing the temperature favours the exothermic formation of hydrogen chloride, so the equilibrium moves proportionately to the right-hand side (more HCl, less H2 or Cl2). If hydrogen chloride is heated to a very high temperature, endothermic direction, then more HCl decomposes into H2 or Cl2.
From rule 2: since there is no net change in the number of moles of gas on reaction, pressure has no effect on the yield of hydrogen chloride and the proportions of HCl, H2 or Cl2 stay the same.
(c) The formation of ammonia
nitrogen + hydrogen ammonia
N2(g) + 3H2(g) 2NH3(g) (plus 92 kJ heat energy, exothermic)
Ammonia formation is favouredby …
Rule 1. Lowering the temperature, because it is an exothermic reaction, but this may make it too slow, compromise required.
Rule 2. Increasing pressure, because there is a reduction in the molecules of gas, but the higher the pressure, the more costly the engineering.
Rule 4. An iron catalyst speeds up the reaction, but has no effect on the % ammonia in the reacted mixture exiting the reactor chamber in the chemical plant of a Haber synthesis process.
In the exam you may have to explain more about the rule and how they are used, which is what this page is all about, plus some more examples below
(d) The manufacture of sulfur trioxide
The process of making sulfur trioxide from sulfur dioxide is one stage in the manufacture of sulfuric acid by the Contact Process.
The equilibrium equation is given below.
2SO2(g) + O2(g) 2SO3(g) (plus 95 kJ heat energy, exothermic)
3 gas molecules ==> 2 gas molecules.
Rule 1. The exothermic reaction is favoured by a lower temperature, but this may be too slow, so a compromise temperature of around 450oC is used, which gives a fast economic rate of sulphur trioxide production.
Rule 2. The reaction is favoured by high pressure (pressure equilibrium rule, 3 => 2 gas molecules, LHS ==> RHS), but only a small increase in pressure is used to give high yields of sulphur trioxide, because the formation of SO3 on the right hand side is so energetically favourable (approx. 99% yield, i.e. only about 1% SO2 unreacted).
Rule 4. The use of the V2O5 catalyst ensures a fast reaction without having to use too a higher temperature which would favour the left hand side and reduce the yield BUT it does not change the % of sulphur trioxide formed, you simply get there faster.
EVALUATION
- How do the equilibrium positions of the following reaction change with pressure?What is the equilibrium constant and the units?
a.N2(g)+3H2(g)=2NH3(g) b.H2(g)+I2(g)=2HI(g)
- State Le Chatellier’s Principle
- Consider the equation for the equilibrium reaction
N2(g)+3H2-2NH3(g) ) ∆H=-92KJmol-1.
Write the equilibrium constant of the reaction.
- Consider the following equilibrium reaction.
X2(g) +2Y(g) –XY2(g) ) ∆H=-52KJmol-1
i.State what happens to the yield of XY2 when the temperature is increased.
ii.Explain the effect of decrease in pressure on the equilibrium position
iii.State the effect of a catalyst position of equilibrium.
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 