A chemistry laboratory is a place, usually a room, where scientific experiments are performed by the use of pieces of apparatus and chemical reagents
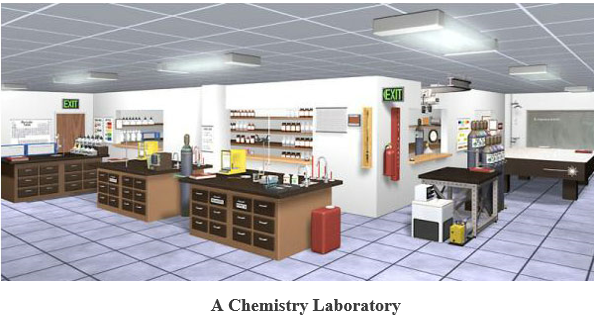
Chemistry laboratory apparatus: Mostly all the apparatus are usually made up of glass such as Pyrex (borosilicate)soda glass, beaker, test tube etc., metals, wood, plastics, and porcelain. Some are improvised i.e. they are locally made such as bamboo for making measuring cylinder, coconut shell for making beaker etc.
EVALUATION
- What is the purpose of laboratory?
- List three materials that can be used to produce laboratory apparatus.
CHEMISTRY LABORATORY APPARATUS AND THEIR USES
- BEAKER: Commonly made of Pyrex glass. It has a flat bottom, cylindrical and graduated, usually with lip for easy pouring.

USES: Beakers are used to keep reagents for chemical tests, for holding and pouring liquids, and for measuring the volume of liquids.
- SEPARATING FUNNEL: It is made of glass with ashort stem, stopcork, and a stopper. It may be conical, cylindrical, or spherical.
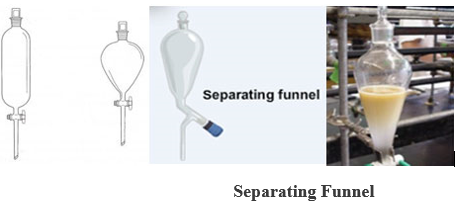
USE: It is used in the separation of immiscible liquids,e.g. a mixture of kerosene and water.
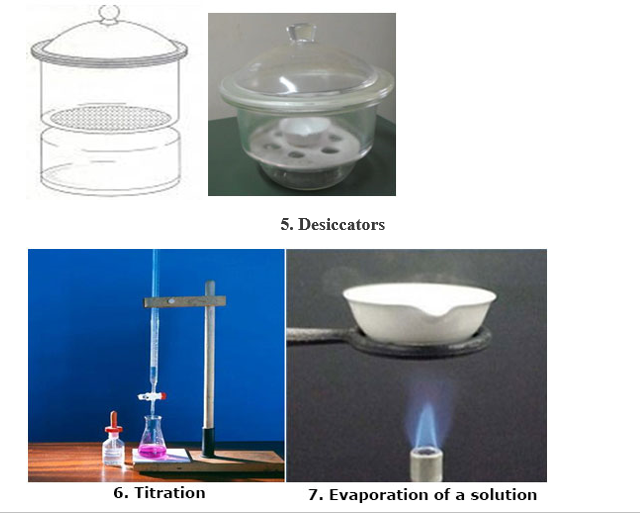
- EVAPORATING BASIN OR DISH: Is made of porcelain. It may be round –bottomed or flat-bottomed, usually shallow, and with spout (lip).
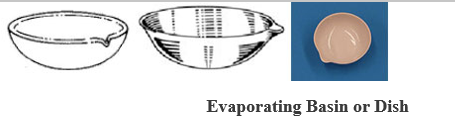
Evaporating Basin or Dish
USES: It is used in the evaporation of a liquid solution to dryness, or to concentrate a solution.
- TRIPOD STAND, WIRE GAUZE AND BUNSEN BURNER:
- TRIPOD STAND: Made of iron, has either a triangular or circular top. It is used as a support for flask when heating.
- WIRE GAUZE: Made of iron mesh with asbestos center. It is usually placed on a tripod stand as a support for flask or boiling tube when heating.
- BUNSEN BURNER: Used in providing heat (high temperature) by the combustion of liquefied gas (propane or butane). It is composed of a metal tube with a wide metal base. It is sometimes positioned in–between the legs of the tripod stand when heating.

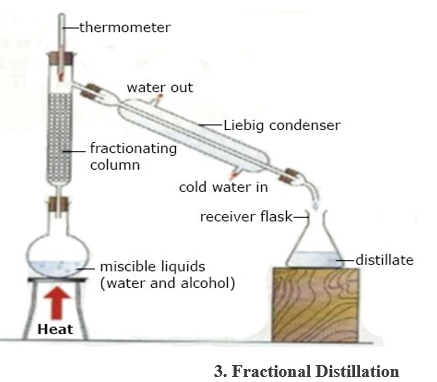
5. DISTILLATION FLASK: has a flat or round bottom made of glass with a slanting side arm.

USE: during distillation.
- MORTAR AND PESTLE:They are made of porcelain or agate.

Mortar and Pestle
USE: They are used in grinding or crushing solids into fine powder.
Chemistry Laboratory Common Equipment
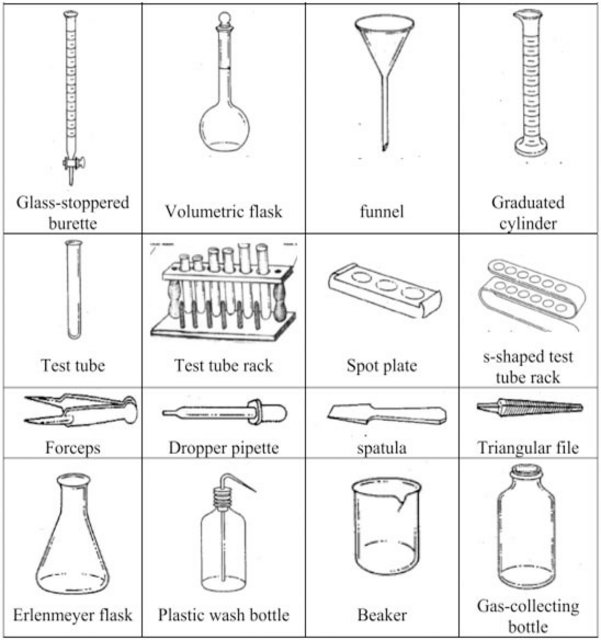
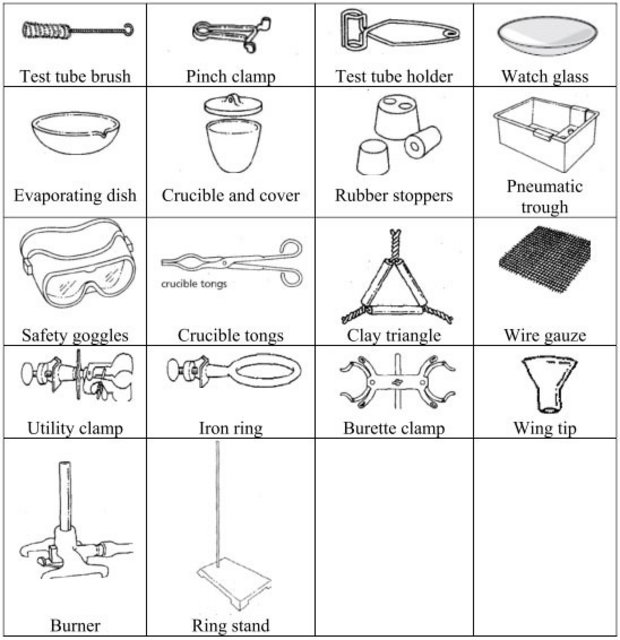
CHEMISTRY LABORATORY SET-UPS
A laboratory set-up is the combination of two or more apparatus, which are arranged in such a manner that the set-up is workable.
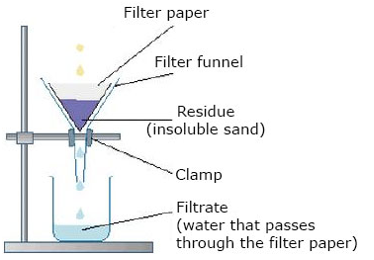
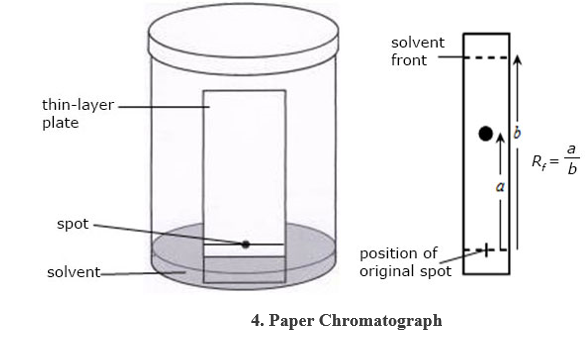
Filteration
COMMON LABORATORY ACCIDENTS
Laboratory accidents are something unpleasant, undesirable or damaging that happens unexpectedly or by chance in the laboratory.
Generally it could involve the inhalation of dangerous substances or physical injury as the case may be. Most of the laboratory accidents occur as follows:
- GLASS CUT:
- Report the situation to your facilitator and let him help the injured person.
- There is always a possibility of infection, even with the most minor injury. For this reason you should report any cut or scrape, even if there is no visible blood.
- If there is blood at any laboratory station, move to your seat in the classroom area until told it is safe to return to the laboratory.
- FIRES:
- Laboratory burners are the source of most problems:
- Bunsen burners have very few malfunctions. If a malfunction occurs, turn off the gas and notify your facilitator – end of problem.
- The flame from alcohol burners is hard to see. Pay close attention when using them.
- Be aware when a burner is in use at your lab station. Be extremely careful during that time.
- Paper is the most common type of fire in the lab.
- This type of fire is caused by carelessness and is easily prevented. Take only one lab sheet to your station to follow your written procedures and record data. Leave all reference materials at your desk. If you need to refer to reference material, leave the lab area to do so.
- If a paper fire occurs, push the paper into the lab sink and turn on the water – end of problem.
- Clothing or Hair is the most dangerous type of fire in the lab.
- Don’t panic!
- If you are the one involved in a fire – stay where you are – help is coming. “Stop, drop, and roll” is still the best course of action. If the fire is not at your lab station – stay away!
THE FIRE EXTINGUISHER IS LOCATED ON THE WEST WALL.
- Only the science facilitator is authorized to use the fire extinguisher.
- Fire extinguishers are classified according to a particular fire type and are given the same letter and symbol classification as that of the fire.

Fire Extinguisher
TYPES OF FIRE EXTINGUISHERS
Most lab fire extinguishers is Type ABC, effective against Types A, B, and C.
The average fire extinguisher only operates about 10 seconds. Do not waste it!
You must get close to the fire – as close as 5 or 6 feet!
To effectively operate an extinguisher, think P-A-S-S.
P — Pull the pin
A — aim the hose at the base of the fire
S — squeeze the handle
S — sweep the hose back and forth
- ACID BURNS
- INHALING TOXIC GASES
- EXPLOSION
- ELECTRIC SHOCK
- SWALLOWING TOXIC CHEMICALS
SAFETY PRECAUTIONS
The following are the basic rules and regulations to guide your safety and hence prevent accidents in the laboratory.
- DANGER
- Always handle glass wares being fragile, with care to avoid glass cuts.
- Never use sodium, potassium, phosphorus or concentrated (conc) acids and alkalis unless you are specially instructed. These chemicals are corrosive. Always add concentrated acid to water slowly, when diluting the acid, never add water to acid. This is to avoid acid burns, explosion and fire.
- Do not taste or drink any chemical, and never smell any chemical directly .This is to avoid swallowing or inhaling toxic chemicals.
- Do not mix chemicals aimlessly, or carry out any experiment except when instructed, to avoid explosion or fire outbreak
- Do not put a glowing splint or a burning paper in the waste bin, to avoid fire outbreak.
- Do not touch or hold any electric instrument with wet hands.
- All accident should be reported immediately to your facilitator.
- Laboratory coats must be worn to protect clothing from soiling, damage from accidents of various sorts.
- CLEANLINESS AND EXPENSES
(i) Pour liquids only down the sink or funnel, and never pour solids, to avoid blockage

(ii) Clean the apparatus after use and replace them in their proper places.
(iii) Wipe down your bench and leave it clean and dry.
(iv) Do not light the Bunsen burner or other sources of heat until required. Turn it off when no longer required.
(v) Do not bring any food or drink in to the laboratory and avoid eating, drinking or smoking in the laboratory.
(vi) Contact lenses should NOT be worn in the laboratory
- It is almost impossible to remove contacts after chemicals have been splashed into the eye.
- Chemicals trapped under contacts will damage the eye even more than normal.
- The plastic used for some types of contact lenses is permeable to vapours found in the laboratory. If these vapours are trapped behind the lens, irritation may occur.
EVALUATION
- You are provided with the following laboratory apparatus give one use for each of the following pieces of apparatus.
(a) Reagent bottle (b) Water bath (c) Combustion boat (d) Wash bottle (e) Deflagratine spoon (f) Evaporating dish (g) Desiccator (h) Tripod stand (i) Test tube rack (j) Liebig condenzer (k) Beam balance.
- With the aid of a diagram, draw the laboratory apparatus/set–up for drying solids in the laboratory.
- 3. Mention THREE basic rules and regulations regarding safety in the laboratory.
- 4. Mention two laboratory accident and they can be prevented.
- 5. Name two apparatus used during filtration process.
- 6. Identify the apparatus you would use to carry out the following in the laboratory
- Separating two immiscible liquids
- Condensing steam to liquid
- Drying a sample of residues
- Measuring a small quantity of liquid
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 