The range of objects and phenomena studied in physics is immense. From the incredibly short lifetime of a nucleus to the age of the Earth, from the tiny sizes of sub-nuclear particles to the vast distance to the edges of the known universe, from the force exerted by a jumping flea to the force between Earth and the Sun, there are enough factors of 10 to challenge the imagination of even the most experienced scientist.
Giving numerical values for physical quantities and equations for physical principles allows us to understand nature much more deeply than does qualitative description alone. To comprehend these vast ranges, we must also have accepted units in which to express them. And we shall find that (even in the potentially mundane discussion of meters, kilograms, and seconds) a profound simplicity of nature appears—all physical quantities can be expressed as combinations of only four fundamental physical quantities: length, mass, time, and electric current.
We define a physical quantity either by specifying how it is measured or by stating how it is calculated from other measurements. For example, we define distance and time by specifying methods for measuring them, whereas we define average speed by stating that it is calculated as distance traveled divided by time of travel.
Measurements of physical quantities are expressed in terms of units, which are standardized values. For example, the length of a race, which is a physical quantity, can be expressed in units of meters (for sprinters) or kilometers (for distance runners).
Without standardized units, it would be extremely difficult for scientists to express and compare measured values in a meaningful way. (See Figure 1.17.)
There are two major systems of units used in the world: SI units (also known as the metric system) and English units (also known as the customary or imperial system). English units were historically used in nations once ruled by the British Empire and are still widely used in the United States. Virtually every other country in the world now uses SI units as the standard; the metric system is also the standard system agreed upon by scientists and mathematicians.
The acronym “SI” is derived from the French Système International.
Table 1.1 gives the fundamental SI units that are used throughout this textbook. This text uses non-SI units in a few applications where they are in very common use, such as the measurement of blood pressure in millimeters of mercury (mm Hg). Whenever non-SI units are discussed, they will be tied to SI units through conversions.
Table 1.1 Fundamental SI Units
| Length | Mass | Time | Electric Current |
| meter (m) | kilogram (kg) | second (s) | ampere (A) |
It is an intriguing fact that some physical quantities are more fundamental than others and that the most fundamental physical quantities can be defined only in terms of the procedure used to measure them. The units in which they are measured are thus called fundamental units. In this textbook, the fundamental physical quantities are taken to be length, mass, time, and electric current. (Note that electric current will not be introduced until much later in this text.)
All other physical quantities, such as force and electric charge, can be expressed as algebraic combinations of length, mass, time, and current (for example, speed is length divided by time); these units are called derived units.
The SI unit for time, the second(abbreviated s), has a long history. For many years it was defined as 1/86,400 of a mean solar day. More recently, a new standard was adopted to gain greater accuracy and to define the second in terms of a non-varying, or constant, physical phenomenon (because the solar day is getting longer due to very gradual slowing of the Earth’s rotation).
Cesium atoms can be made to vibrate in a very steady way, and these vibrations can be readily observed and counted. In 1967 the second was redefined as the time required for 9,192,631,770 of these vibrations. (See Figure 1.18.)

Accuracy in the fundamental units is essential, because all measurements are ultimately expressed in terms of fundamental units and can be no more accurate than are the fundamental units themselves.
The SI unit for length is the meter (abbreviated m); its definition has also changed over time to become more accurate and precise. The meter was first defined in 1791 as 1/10,000,000 of the distance from the equator to the North Pole. This measurement was improved in 1889 by redefining the meter to be the distance between two engraved lines on a platinum-iridium bar now kept near Paris.
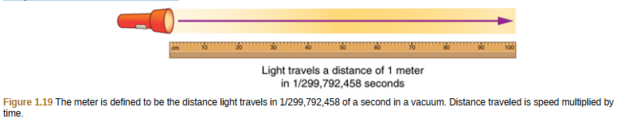
By 1960, it had become possible to define the meter even more accurately in terms of the wavelength of light, so it was again redefined as 1,650,763.73 wavelengths of orange light emitted by krypton atoms. In 1983, the meter was given its present definition (partly for greater accuracy) as the distance light travels in a vacuum in 1/299,792,458 of a second. (See Figure 1.19.)
This change defines the speed of light to be exactly 299,792,458 meters per second. The length of the meter
will change if the speed of light is someday measured with greater accuracy.
The SI unit for mass is the kilogram (abbreviated kg); it is defined to be the mass of a platinum-iridium cylinder kept with the old meter standard at the International Bureau of Weights and Measures near Paris. Exact replicas of the standard kilogram are also kept at the United States’ National Institute of Standards and Technology, or NIST, located in Gaithersburg, Maryland outside of Washington D.C., and at other locations around the world. The determination of all other masses can be ultimately traced to a comparison with the standard mass.
Electric current and its accompanying unit, the ampere, will be introduced in Introduction to Electric Current, Resistance, and Ohm’s Law when electricity and magnetism are covered. The initial modules in this textbook are concerned with mechanics, fluids, heat, and waves. In these subjects all pertinent physical quantities can be expressed in terms of the fundamental units of length, mass, and time.
Table 1.2 Metric Prefixes for Powers of 10 and their Symbols
| Prefix | Symbol | Value[1] | Example (some are approximate) | |||
| exa | E | 1018 | exameter | Em | 1018 m | distance light travels in a century |
| peta | P | 1015 | petasecond | Ps | 1015 s | 30 million years |
| tera | T | 1012 | terawatt | TW | 1012 W | powerful laser output |
| giga | G | 109 | gigahertz | GHz | 109 Hz | a microwave frequency |
| mega | M | 106 | megacurie | MCi | 106 Ci | high radioactivity |
| kilo | k | 103 | kilometer | km | 103 m | about 6/10 mile |
| hecto | h | 102 | hectoliter | hL | 102 L | 26 gallons |
| deka | da | 101 | dekagram | dag | 101 g | teaspoon of butter |
| — | — | 100 (=1) | ||||
| deci | d | 10-1 | deciliter | dL | 10-1 L | less than half a soda |
| centi | c | 10-2 | centimeter | cm | 10-2 m | fingertip thickness |
| milli | m | 10-3 | millimeter | mm | 10-3 m | flea at its shoulders |
| micro | µ | 10-6 | micrometer | µm | 10-6 m | detail in microscope |
| nano | n | 10-9 | nanogram | ng | 10-9 g | small speck of dust |
| pico | p | 10-12 | picofarad | pF | 10-12 F | small capacitor in radio |
| femto | f | 10-15 | femtometer | fm | 10-15 m | size of a proton |
| atto | a | 10-18 | attosecond | as | 10-18 s | time light crosses an atom |
See other derived units and their physical quantity below
| QUATITY | DERIVATION | UNIT |
| Area | Length × breadth | M2 |
| Volume | Length × breadth x height | M3 |
| Density | Mass/volume | Kgm-3 |
| Velocity | Displacement/time | Ms-1 |
| Acceleration | change in velocity/time | Ms-2 |
| Weight | Mass x acceleration due gravity | Newton (N) |
| Momentum | Mass x velocity | Newton second (Ns) |
| Pressure | Force/ area | Pascal or Nm-2 |
| Energy or work | Force X distance | Joule (j) or Ns |
| Power | Work/time | Watt (w) or js-1 Or NmS-1 |
This OpenStax book is available for free at http://cnx.org/content/col11406/1.14
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 