Based on source of pollution, the following types of water pollution are recognised
Based on severity of pollution, the water pollution could be classified as
Quite frequently, a water pollution could find a place in two or more groups. For e.g., an agricultural water pollution may be serious and industrial water pollution may be mild or sea water may be polluted by agricultural chemical and underground water may be polluted by city wastes.
Although all the above groupings are meaningful, it is more convenient if the classification based on utility and location of water is considered for detailed discussion. In this classification, drinking water pollution can be caused by its source and during its path of movement. Thus, the ground water pollution and river water pollution may ultimately lead to drinking water pollution when ground water or river is polluted. River water and underground water can lead to many other complications other than drinking water pollution alone. It is to be observed that sea water is significantly polluted, although no pollutant is directly added to sea (except accidental ship leakage or sinking of ship etc). This is because, all polluted rivers lead to pollutants to sea and due to continuous accumulation, the concentration of pollutants in sea water gets increased as the time passes.
Current water pollution issues can be divided into the following categories:
Oxygen-consuming wastes comprise of raw sewage from municipalities and other wastes from paper mills, tanneries, food-processing plants and agriculture; these cause a proliferation of aerobic decomposers who consume dissolved oxygen (DO) in water more rapidly than it can be replaced by the atmosphere. Organic matter undergoes oxidation in rivers under the action of micro-organisms.
Biochemical oxygen demand (BOD) is a measure of demand for oxygen utilized by micro-organism, during oxidation of organic matter. It is defined as the amount of oxygen in milligram per liter or in ppm used by micro-organisms to degrade the organic matter. A high BOD value indicates more polluted water.
The dissolved oxygen in rivers and other water bodies is usually sufficient to decompose only small or treated discharge of such waste. The products formed during aerobic decomposition e.g. CO2, SO42-, NH4+ or NO3– ions do not cause much pollution.
If, however, the amount of sewage and other wastes are heavy, then the dissolved oxygen (DO) is insufficient and oxidation of organic matter is incomplete. Incomplete oxidation results in the formation of amines, ethane, H2S, etc., which produce wormy smell. It also depletes the dissolved oxygen (DO) in water to levels, which cannot sustain aquatic life. If the level of dissolved O2 falls below 5 ppm (parts per million), fish begin to die.
When we treat our sewage with chlorine to kill bacteria, we also kill marine ecosystems with the chlorine itself, or with carcinogenic chlorinated hydrocarbon by products.
These are the various pathogenic micro-organisms which may enter the water along with sewage or other wastes. These microbes, mainly bacteria and viruses, can cause various diseases such as cholera, typhoid, dysentery, gastroenteritis, polio, hepatitis etc. in the water supply drawn from these sources. The bacterium found in sewage (E-coli) has been known to affect shellfish and salmon populations in USA and causes severe food poisoning and gastroenteritis.
The presence of plant nutrients in lakes and slow-moving waters supports high population of aquatic plants that on decay, deplete dissolved oxygen (DO) making the survival of aquatic life problematic. Besides this, these dead plants also produce disagreeable odor.
The enrichment of water by nutrients is known as ‘eutrophication’. Lakes and slow-moving waters age through eutrophication and over periods of several millennia, they get converted into swamps and marshes.
Pesticides, detergents, chemical dyes and other industrial chemicals and their waste constitute synthetic organic compounds. When present in water, these chemicals can act as toxic poisons for plants, animals and humans. Such chemicals enter the hydrosphere either by usage, by accidental or intentional disposal of wastes from manufacturing units and by losses during their transport. In the USA at the turn of the century, lobster die-off occurred on Long Island. It may be linked to the runoff of the pesticide malathion, used to eradicate the West Nile virus-carrying mosquitoes, since crustaceans are closely related to insects.
Some laundry detergents and fertilizers such as nitrogen, phosphorus and other nutrients cause an overgrowth of algae, bacteria, and aquatic plants. This also leads to ‘eutrophication’ and eventually the lake can no longer support life.
The oxides of nitrogen and sulphur are corrosive and poisonous. When in excess in the atmosphere, these gases react with water (e.g., rain water) to form acids, and thus result into acid rain.
Acid rain has the following harmful effects:
Chemistry of acid rain
Normally while rain travels through the air, it dissolves floating chemicals and washes down particles that are suspended in air. At the start of its journey raindrops are neutral (pH = 7). In clean air, rain picks up materials that occur naturally such as dust, pollen, some CO2 and other chemicals produced by lightening or volcanic activities. These substances make rain slightly acidic (pH = 6), which is not dangerous. However, when rain falls through polluted air, it comes across chemicals such as gaseous oxides of sulphur (SOx), oxides of nitrogen (NOx), mists of acids such as hydrochloric and phosphoric acid, released from automobile exhausts industrial plants, electric power plants etc.
These substances dissolve in falling rain making it more acidic than normal with pH range between 5.6 -3.5. In some case, it’s pH gets lowered to the extent of 2. This leads to acid rain. The term acid rain is used here to describe all types of precipitation, namely, rain, snow, fog and dew more acidic than normal.
In the natural processes of volcanic eruptions, forest fires and bacterial decomposition of organic oxides of sulphur and nitrogen, production and reductions of gases naturally tend to an equilibrium. Power plants, smelting plants, industrial plants, burning of coal and automobile exhausts, release additional sulphur dioxide, nitrogen oxides and acidic soot, causing pollution. Sulphur dioxide and nitrogen dioxide interact with water vapours in presence of sunlight to form sulphuric acid and nitric acid mist.
SO2 + H2O → H2SO3
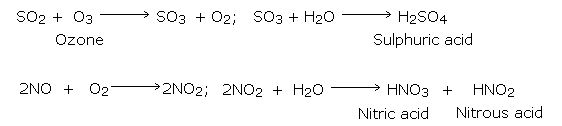
The formed sulphuric acid and nitric acid remain as vapour at high temperatures. These begin to condense as the temperature falls and mix with rain or snow, on the way down to the Earth and make rain sufficiently acidic.
SOx, NOx mixed with water as acid rain causes plant, animal and material damage. Some of the significant ill effects of acid rain are:
Acid rain chemically strips waterways of necessary nutrients and lowers the pH to levels where plants and animals cannot live. Most of the aquatic animals cannot survive when the pH is less than 4. Some species of fish, such as salmon, die even when the pH is less than 5.5. Certain species of algae and zooplankton are eliminated at pH less than 6. A reduction in the zooplankton and bottom fauna ultimately affects the food availability for the fish population. The problem is most severe downwind of industrial areas where fishing and tourism are major sources of income such as in Norway and Sweden.
Acidic water is dangerous to plants. Sulphuric and nitric acid rain washes nutrients out of the soil, damages the bark and leaves of trees and harms the fine root hairs of many plants which are needed to absorb water. Leaf pigments are decolorized because acid affects green pigment (chlorophyll) of plants. Agricultural productivity is also decreased. Several non-woody plants, such as barley, cotton and fruit trees like apple, pear, etc., are severely affected by acid rain. Since the acid concentration increases near the base of clouds by density, high altitude trees and vegetation may be exposed to pH levels as low as 3. Unique areas such as the Black Forest in Germany and sugar maples in Vermont (USA) are particularly threatened.
Metallic surfaces exposed to acid rain are easily corroded. Textile fabrics, paper and leather products lose their material strength or disintegrate by acid rain.
Building materials such as limestone, marble, dolomite, mortar and slate are weakened on reaction with acid rains because of the formation of soluble compounds.
CaCO3 (s) + H2SO4 (aq) → H2O (l) + CO2 (g) + CaSO4 (s)
Thus, acid rain is dangerous for historical monuments.
Water is so valuable to the entire system of the human body that it is wise to use only the Best. Use pure steam distilled water for health and well being.
REVISION EXERCISES (Post answers using question box below for evaluation and discussion. Add Question title)
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 