Energy is conserved in chemical reactions. One way of stating the ‘law of Conservation of Energy‘ is to say the amount of energy in the universe at the end of a chemical reaction is the same as before the reaction took place. If a reaction transfers energy to the surroundings the product molecules must have less energy than the reactants, by the amount transferred. Conversely, if a reaction absorbs energy from the surroundings, they must have less energy, and the products must have more energy. An exothermic chemical reaction transfers energy to the surroundings, usually given out in the form of heat energy, so raising the temperature of the surroundings. Therefore the products have less energy than the reactants and the surroundings have more energy. |
Exothermic reactions include combustion of fuels, many oxidation reactions, acid-alkali neutralisation reactions, reactive metals with water,
moderately reactive metals with strong acids.
Exothermic reactions are used in self-heating cans and hand warmers.
An endothermic chemical reaction absorbs energy from the surrounding, usually in the form of heat energy, so cooling the surroundings, but sometimes the system is heated to provide the heat energy and a high enough temperature to promote the reaction. This means the products have more energy than the reactants and the surroundings have less energy.
Endothermic reactions include thermal decomposition of compounds e.g. carbonates, the reaction between citric acid and sodium hydrogencarbonate, sports injury packs to produce cooling effects
Why is it important to know about energy changes in chemical reactions?
Its important to know how much energy fuels release on combustion i.e. their calorific value.
Its important to know the energy released on burning petrol. diesel, coal or any other fossil fuel and alternative fuels like hydrogen or biofuels (biomass fuels).
The same sort of data is important in knowing how much energy is released on metabolising foods such as fats and carbohydrates.
Accurate energy change data is important in managing chemical processes in industry.
Exothermic reactions may provide their own heat if the process is carried out at high temperatures, energy transfer data provides some of the information needed.
Conversely, excess heat from an exothermic reaction may have to be removed using heat exchangers to avoid ‘overheating’ and excessive reaction rates that could be dangerous. If gases are involved, lack of control could lead to a build up of pressure resulting in an explosion.
Endothermic chemical processes often need a high temperature to promote
the absorption of heat energy, otherwise the reaction rate might economically far too slow. The amount of energy needed can be calculated from energy transfer data.
Heat Changes in Chemical Reactions
When chemical reactions occur, as well as the formation of the products – the chemical change, there is also a heat energy change which can often be detected as a temperature change.
This means the products have a different energy content than the original reactants (see the reaction profile diagrams below).
If the products contain less energy than the reactants, heat is released or given out to the surroundings and the change is called an exothermic reaction (exothermic energy transfer, exothermic energy change of the system).
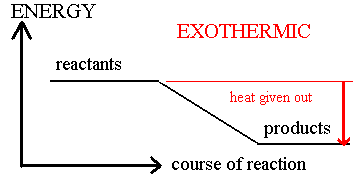
This is illustrated by the simple energy level diagram above for an exothermic reaction.
The products have less energy than the original reactants (lower energy level) and the difference comes out as heat energy released to the surroundings.
The difference in heights of the energy levels tells you how much energy is released in an exothermic reaction.
The temperature of the system will be observed to rise in an exothermic change.
transfer, So an exothermic reaction is one which gives out energy to the surroundings, usually in the form of heat energy, hence the rise in temperature.
Examples of exothermic reactions:
The burning or combustion of hydrocarbon fuels e.g. petrol or candle wax, these are very exothermic reactions.
1.The exothermic burning-combustion of fossil fuels is very important source of energy.
2.methane (natural gas) + oxygen ==> carbon dioxide + water (+ heat energy)
CH4 + 2O2 ==> CO2 + 2H2O
3.The burning of magnesium, reaction of magnesium with acids, or the reaction of sodium with water
2Mg + O2 ==> 2MgO (+ heat energy)
4.The neutralisation of acids with alkalis
Using hydrogen as a fuel in hydrogen-oxygen fuel cells xplosions are caused by VERY fast exothermic reactions producing very fast large expanding volumes of gases.
Other uses of exothermic reactions:
Hand warmers contain chemicals that when mixed together give out heat.
Self-heating cans of coffee, soup or hot chocolate have chemicals contained in the base of the container that when mixed generate enough energy to heat the contents of the can.
If the products contain more energy than the reactants, heat is taken in or absorbed from the surroundings and the change is called an endothermic reaction (endothermic energy endothermic energy change of the system).

This is illustrated by the simple energy level diagram above for an endothermic reaction.
The products have more energy than the original reactants (higher energy level) and the difference comes out as heat energy absorbed from the surroundings.
The difference in heights of the energy levels tells you how much energy is absorbed in an endothermic reaction.
If the change can take place spontaneously, the temperature of the reacting system will fall but, as is more likely, the reactants must be heated to speed up the reaction and provide the absorbed heat.
So an endothermic reaction is one which absorbs energy from the surroundings, usually in the form of heat, hence the observed fall in temperature in some reaction.
Examples of endothermic reactions
The thermal decomposition of limestone
calcium carbonate (limestone) ==> calcium oxide (lime) + carbon dioxide
CaCO3 (+ heat energy) ==>CaO + CO2
This only happens at temperatures above 900oC.
the cracking of oil fractions
e.g. octane (+ heat energy) ==> hexane + ethene
C8H18 ==> C6H14 + C2H4
Again this needs a very high temperature AND a catalyst too.
These are two very important endothermic reactions used in the chemical industry.
Dissolving ammonium nitrate in water doesn’t need heating, the salt spontaneously dissolves and the temperature of the water/solution immediately falls as energy from the surroundings is absorbed, in fact from the water itself.
Other uses of endothermic reactions:
Some sports injury packs have a mixture of chemicals (or maybe a salt and water) that when mixed undergo an endothermic energy change, thereby absorbing heat from the surroundings, cooling some poor bruised limb! Rather more convenient and less messy than packs of ice!
One of the most important endothermic reactions, for which most of animal life depends is photosynthesis.
The energy from sunlight is absorbed as water and carbon dioxide are converted to glucose and oxygen.
6H2O + 6CO2 + sunlight energy ==> C6H12O6 + 6O2
However, on ‘burning’ the glucose/carbohydrates in our bodies, the ‘stored’ sunlight energy is released to keep us warm and drive all the chemical processes in our cells, so the opposite reaction is exothermic!
C6H12O6 + 6O2 ==> 6H2O(l) + 6CO2 + heat/chemical energy
The difference between the energy levels of the reactants and products gives the overall energy change for the reaction
At a more advanced level the heat change is called the enthalpy change is denoted by delta H, ΔH.
ΔH is negative (-ve) for exothermic reactions i.e. heat energy is given out and lost from the system to the surroundings which warm up.
ΔH is positive (+ve) for endothermic reactions i.e. heat energy is gained by the system and taken in from the surroundings which cool down OR, as is more likely, the system is heated to provide the energy needed to effect the change.

Extra NOTE: In some exothermic changes, no heat is not released e.g. in batteries and fuel cells, where the energy is released as electrical energy.
Reversible Reactions and energy changes
If the direction of a reversible reaction is changed, the energy change is also reversed.
For a reversible reaction, the energy released in the exothermic reaction is numerically equal to the heat absorbed in the reverse reaction.
For example: the thermal decomposition of hydrated copper(II) sulphate is a very good example to observe in the school laboratory, even though it is not practical to measure the actual energy changes involved.
blue hydrated copper(II) sulphate + heat white anhydrous copper(II) sulphate + water
CuSO4.5H2O(s) CuSO4(s) + 5H2O(g)
On heating the blue solid, hydrated copper(II) sulphate, steam is given off and the white solid of anhydrous copper(II) sulphate is formed and left as the residue.
This is a thermal decomposition and is endothermic as heat is absorbed (taken in) from the surroundings.
The energy is needed to break down the crystal structure and drive off the water.
When the white solid is cooled and a few drops of water added, blue hydrated copper(II) sulphate is reformed and heat energy is given out to the surroundings, the mixture hots up!
The reverse reaction is exothermic as heat is given out.
i.e. on adding water to white anhydrous copper(II) sulphate the mixture heats up as the blue crystals reform.
Water molecules recombine with the copper ion releasing energy when the new bonds are formed.
Types of Chemical Reactions
Chemical reactions are processes in which substances change into other substances.
You know a chemical reaction takes place if one or more of these occur:
same. Sometimes the difference is great and can be easily detected. At other times, the difference is slight and more difficult to detect.
reaction of solids is much harder to detect.
Most chemical reactions can be placed into one of five basic types:
1. Decomposition Reactions
Some decomposition complications with heat:
H2SO3 → SO2 + H2O
Ca(OH)2 → CaO + H2O
Li2CO3 → Li2O + CO2
Metallic chlorates, when heated, decompose into metallic chlorides and O2.
| 2KClO3 → 2KCl + 3O2 2. Synthesis Reactions
3. Single Displacement Reactions
4. Double Displacement Reactions
|
5. Combustion Reactions
|
The rusting of iron – 4Fe + 3O2 → 2Fe2O3
Chemical reactions can be classified in other ways as well:
Neutralization Reactions
Mg(OH)2 (s) + 2HCl (aq) → MgCl2 (aq) + 2H2O (l)
Oxidation-Reduction Reactions
S + O2 → SO2
Precipitation Reactions
2KI (aq) + Pb(NO3)2 (aq) → 2KNO3 (aq) + PbI2 (s)
The physical state symbol (aq) says the reaction is taking place in a water solution. The physical state symbol (s) says the lead (II) iodide is a solid – therefore insoluble
FIRST AND SECOND LAW OF THERMODYNAMICS.
Thermodynamics is a branch of physics which deals with the energy and work of a system. Thermodynamics deals only with the large scale response of a system which we can observe and measure in experiments. In aerodynamics, the thermodynamics of a gas obviously plays an important role in the analysis of propulsion systems but also in the understanding of high speed flows. The first law of thermodynamics defines the relationship between the various forms of energy present in a system (kinetic and potential), the work which the system performs and the transfer of heat. The first law states that energy is conserved in all thermodynamic processes.
The First Law of Thermodynamics – Energy is conserved.
The internal energy of a system is the sum of all the kinetic and potential energies of all its components.
ΔE = Efinal − Einitial
The equation above indicates the change in internal energy, ΔE, is the difference between the final energy, Efinal, and the initial energy, Einitial.
Thermodynamic quantities like ΔE have three parts: a number, a unit, and a sign.
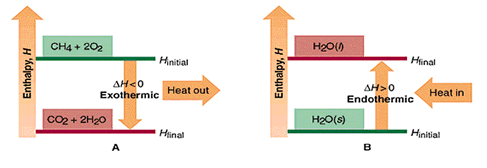
In a chemical reaction the initial state of the system refers to the reactants and the final state refers to the products.
2 H2(g) + O2(g) → 2 H2O(g)
In the reaction above, the system loses energy to the surroundings as heat. Because heat is lost from the system, the internal energy of the products (final state) is less than that of the reactants (initial state), and ΔE is negative.
Another way of saying this – as heat is added to or removed from a system, work is done on or by the system.
Using q to represent the heat added to or removed from the system, and w to represent work, the First Law of Thermodynamics can be represented by the equation:
ΔE = q + w
For q
For w
For ΔE
When a process (such as a chemical reaction) occurs in which the system absorbs heat, the process is called endothermic.
A process in which the system loses heat is called exothermic.
The work involved in the expansion or compression of gases is called pressure-volume work, or P-V work.
When the pressure is constant, the sign and magnitude of the P-V work is given by
w = − P ΔV
where P is pressure and ΔV is the change in volume of the system
(ΔV = Vfinal − Vinitial).
Enthalpy, H is the thermodynamic function that accounts for heat flow in processes occurring at constant pressure when no forms of work are performed other than P-V work.
H = E + PV
At constant pressure, a change in enthalpy equals the change in internal energy plus the product of the constant pressure times the change in volume.
ΔH = ΔE + P ΔV
Enthalpy of reaction, the enthalpy change for a chemical reaction is expressed by the equation: ΔH = Hproducts − Hreactants Here is an example of a thermochemical equation: 2 H2(g) + O2(g) → 2 H2O(g) ΔH = − 483.6 kJ
This equation indicates two moles of hydrogen gas burn to form two moles of water at a constant pressure, releasing 483.6 kJ of heat.
We can imagine thermodynamic processes which conserve energy but which never occur in nature. For example, if we bring a hot object into contact with a cold object, we observe that the hot object cools down and the cold object heats up until an equilibrium is reached. The transfer of heat goes from the hot object to the cold object. We can imagine a system, however, in which the heat is instead transferred from the cold object to the hot object, and such a system does not violate the first law of thermodynamics. The cold object gets colder and the hot object gets hotter, but energy is conserved. Obviously we don’t encounter such a system in nature and to explain this and similar observations, thermodynamicists proposed a second law of thermodynamics. Clasius, Kelvin, and Carnot proposed various forms of the second law to describe the particular physics problem that each was studying. The description of the second law stated on this slide was taken from Halliday and Resnick’s textbook, “Physics“. It begins with the definition of a new state variable called entropy. Entropy has a variety of physical interpretations, including the statistical disorder of the system, but for our purposes, let us consider entropy to be just another property of the system, like enthalpy or temperature. The second law states that there exists a useful state variable called entropy S. The change in entropy delta S is equal to the heat transfer delta Q divided by the temperature T.
For a given physical process, the combined entropy of the system and the environment remains a constant if the process can be reversed. If we denote the initial and final states of the system by “i” and “f”: Sf = Si (reversible process) An example of a reversible process is ideally forcing a flow through a constricted pipe. Ideal means no boundary layer losses. As the flow moves through the constriction, the pressure, temperature and velocity change, but these variables return to their original values downstream of the constriction. The state of the gas returns to its original conditions and the change of entropy of the system is zero. Engineers call such a process an isentropic process. Isentropic means constant entropy. The second law states that if the physical process is irreversible, the combined entropy of the system and the environment must increase. The final entropy must be greater than the initial entropy for an irreversible process: Sf > Si (irreversible process) An example of an irreversible process is the problem discussed in the second paragraph. A hot object is put in contact with a cold object. Eventually, they both achieve the same equilibrium temperature. If we then separate the objects they remain at the equilibrium temperature and do not naturally return to their original temperatures. The process of bringing them to the same temperature is irreversible. EVALUATION
4.When change in H is negative, the reaction is said to be a. exothermic b. endothermic
section b 1.Draw the energy diagrams for the catalysed and uncatalysed i Exothermic reaction ii Endothermic reaction 2. Give 5 examples each of Exothermic and Endothermic Reactions (post your answers on the forum for review) |
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 