Radioactivity refers to the particles which are emitted from nuclei as a result of nuclear instability. Because the nucleus experiences the intense conflict between the two stronger forces in nature, it should not be surprising that there are many nuclear isotopes which are unstable and emit some kind of radiation. The most common types of radiation are called alpha, beta and gamma radiation, but there are several other varieties of radioactive decay.
Alpha Radioactivity
Composed of two protons and two neutrons, the alpha particle is a nucleus of the element helium. Because of its very large mass (more than 7000 times the mass of the beta particle) and its charge, it has a very short range. It is not sustainable for radiation therapy since its range is less than a tenth of a millimeter inside the body. Its main radiation hazard comes when it is ingested into the body; it has great destructive power within its short range. In contact with fast-growing membranes and living cells, it is positioned for maximum damage.
Alpha particle emission is modeled as a barrier penetration process. The alpha particle is the nucleus of the helium atom and is the nucleus of highest stability.
Alpha Barrier Penetration
The energy of emitted alpha particles was a mystery to early investigators because it was evident that they did not have enough energy, according to classical physics, to escape the nucleus. Once an approximate size of the nucleus was obtained by Rutherford scattering, one could calculate the height of the Coulomb barrier at the radius of the nucleus. It was evident that this energy was several times higher than the observed alpha particle energies. There was also an incredible range of half lives for the alpha particle which could not be explained by anything in classical physics.

The resolution of this dilemma came with the realization that there was a finite probability that the alpha particle could penetrate the wall by quantum mechanical tunneling. Using tunneling, Gamow was able to calculate a dependence for the half-life as a function of alpha particle energy which was in agreement with experimental observations.
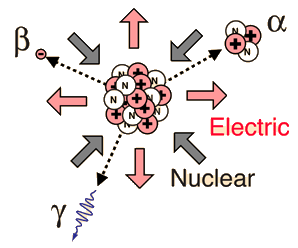
Alpha, Beta, and Gamma
Historically, the products of radioactivity were called alpha, beta, and gamma when it was found that they could be analyzed into three distinct species by either a magnetic field or an electric field.
Penetration of Matter
Though the most massive and most energetic of radioactive emissions, the alpha particle is the shortest in range because of its strong interaction with matter. The electromagnetic gamma ray is extremely penetrating, even penetrating considerable thicknesses of concrete. The electron of beta radioactivity strongly interacts with matter and has a short range.

Different radiations have different properties, as summarized below:
Properties of Radiation
| Radiation | Alpha (α) –particles | Beta particles | Gamma (λ) rays |
| Nature | Helium nuclei 42H | High energy electrons | Electromagnetic waves of very short wavelength |
| Velocity |
Charge
5-7% speed of light
+2e(+3.2 x 10-19 C)Travel at approx speed of light –e(-1.6 x 10-19 C)Travel at speed of light
Electrically neutralMassRelatively massiveRelatively lightNegligibleEffect of magnetic fieldSlightly deflected in a magnetic field in a direction expected for a +ve chargeStrongly deflected in a magnetic field in a direction expected for a -ve chargeSmall or no effectIonizing powerLarge, cause heavy ionizationMedium. About 0.1% of that of α-particlesSmallPenetrating powerLittle penetrating power e.g. by thin sheets of paperGood penetrating power in air, , several mm of aluminiumHigh penetrating power in air and in solid e.g. many cm of leadFlourescentCause fluorescence in ZnSNo fluorescence in ZnS
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 