CONTENT
-Source
-General molecular formula
-Nomenclature
– Classification
-Types, Preparation, Properties and Uses.
Test for Alkanols
SOURCES OF ALKANOLS
– From destructive distillation wood.
– From starchy food and sugar
General molecular formular
Alkanol is a homologous series with general molecular formular of Cn H2n+1OH or ROH.
Or (CnH2n+2O).
Nomenclature:
The names of alkanols are obtained by substituting “e” in alkanes with “Ol” in alkanol e.g. methanol
(CH3OH), ethanol (CH3CH2OH).
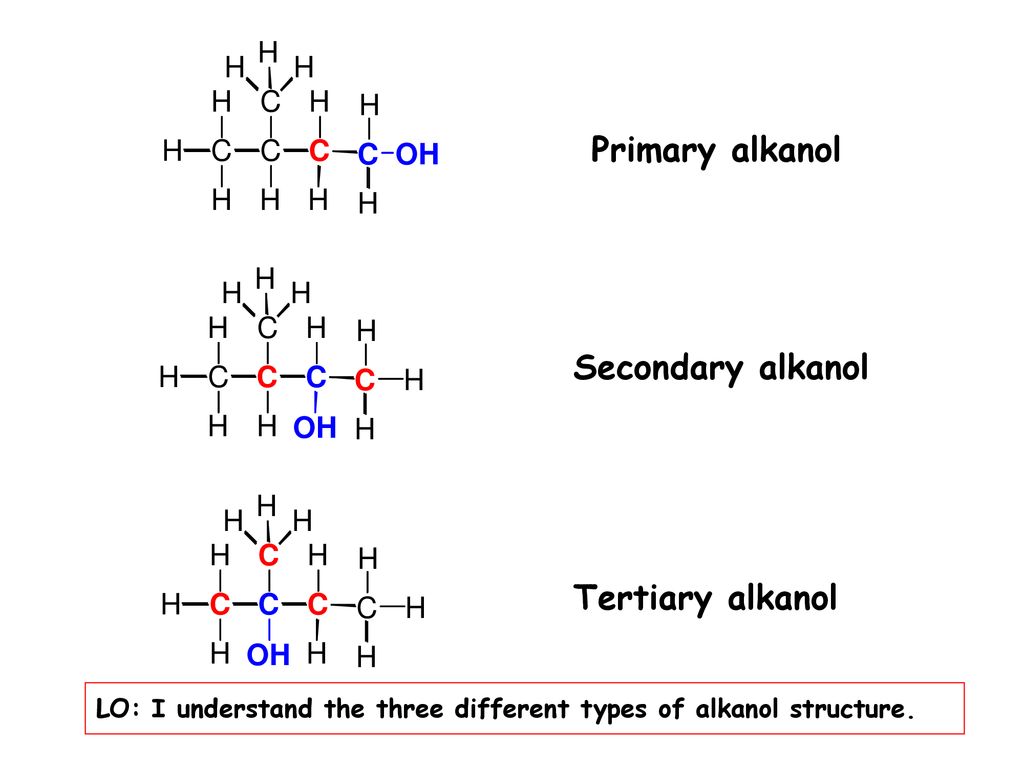
Classification
The alkanols are classified based on the number of alkyl groups directly linked to the carbon atom
holding the hydroxyl group.

N.B: Examine the structure of the alkanol, identify the carbon atom bonded to the –OH, count the number of alkyl groups that are directly attached to the carbon atom. Then you can say whether it is 10, 20 or 30.
EVALUATION
1. Name the functional group in the alkanol.
2. Give an example each by writing the structure and names of the classes of alkanols.
Types of Alkanol
The type of alkanols is determined by the number of the hydroxyl group –OH, present in the molecule.
PREPARATION e.g. ethanol
1. Laboratory preparation
– Hydrolyzing ethyl esters with hot alkali
– Reducing ethanol with nascent hydrogen
2. Commercial preparation
– From ethene
Ethene is obtained by the cracking of petroleum. It is then absorbed in 95% H2SO4 at 800C and
30 atoms to form C2H5HSO4
C2H4 + H2SO4 → C2H3HSO4
The C2H3HSO4 is bedded in water to produce ethanol; C2H3HSO4 + H2O → C2H3OH + H2SO4.
The ethanol is distilled off leaving the acid behind which can be used again.
-Preparation by fermentation ethanol is prepared industrially from raw materials containing
starch or sugar by the process of fermentation.
-The fermentation is an enzymatic process which involves the decomposition of large organic
molecules to simple molecule by micro-organism.
-The common micro-organism used is YEAST
EVALUATION
1. Name three (3) types of alkanol and give one example each.
2. State two methods by which ethanol can be prepared industrially.
CHEMICAL PROPERTIES
1. Combustion: the lower members of alkanols burn with clean flames in plenty air
2CH3OH + 3O2 → 2CO2 4 +H2O
2. Oxidation
The products of oxidation depend on the structure of the alkanol.
USES OF ETHANOL
1. It is used as organic solvent
2. It is the main constituent of methylated spirit used to clean wounds and to dissolve paint.
3. It is used as petrol addictive for use as fuel in vehicles.
4. Used to manufacture other chemicals such as ethanol and ethanoic acid.
5. It is used as ingredient in making alcoholic drinks e.g. beers, wines and spirits.
6. It is used as anti-freeze in automobile radiator because of its low freezing point (-1170C).
TEST FOR ALKANOLS
– It librates hydrogen gas on reacting with sodium methal.
– It changes to alkanoic acid (primary alkanol) on exposure to air or reacting with oxidizing agent.
EVALUATION
1. State five (5) chemical properties of ethanol.
2a. Identify two (2) physical properties of ethanol
b. State two (2) uses of alkannol.
READING ASSIGNMENT
– New School Chemistry By O.Y. Osei Yaw Ababio pages 496-501
WEEKEND ASSIGNMENT
1. The functional groups of the alkanol is
A. CnH2n + 1 OH B. carboxylic group C. hydroxyl group D. CnH2n+ 2
2. Primary alkanols are oxidized to carbonylic acid, secondary alkanols are oxidized to alkanones while tertiary alkanols are A. oxidized to alkanols B. oxidized to alkanones C. not oxidized D. oxidized to alkene
3. The soluble of alkanols in water is due to
A. the covalent nature B. hydrogen bonding C. their low melting point
D. their low melting point
4. When acidified KMnO4 is used as oxidizing agent for alkanol, the colour change observed is
A. yellow to red B. purple to colourless C. orange to green D. white to black
5. Which of the following enzymes converts glucose to ethanol?
A. maltose B. zymase C. diastase D. amylase
THEORY
1(a) Write the structural formula of two named secondary alkanols.
(b) Explain the structural difference between primary and tertiary alkanols.
2(a) What is fermentation?
(b) Describe the preparation of ethanol from table sugar.
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 