AMINES:
It has a functional group of NH2.
PHYSICAL PROPERTIES
1. They can dissolve in water.
2. They are gases and liquid.
3. They have fishy odour.
CHEMICAL PROPERTIES
1. As bases they neutralize acids.
2. They dissociate/ionize in water e.g. CH3NH2 + H2O CH3NH3++ OH–
USES
1. Used in making nylon
2. They can also be used in making polyamide.
EVALUATION
1. State two (2) physical properties and two (2) chemical properties of amine.
2. Give the classes of amine according to the number of their alkyl groups.
AMIDES
O
It has functional group of –C which is known as carbonamide group.
PHYSICAL PROPERTIES:
1. Only methanamide is a liquid while others are solid.
2. They have high melting point and high boiling point.
CHEMICAL PROPERTIES:
1. Amide can be hydrolysed in the presence of alkali and mineral acid e.g.
CH3CONH2 + H2O → CH3COOH + NH3
2. In the presence of sodium hydroxide and bromine, amides produce amines with elimination of one carbonyl group.
e.g.CH3CONH2 + Br2 + 4NaOH → CH3NH2 + 2NaBr + Na2CO3 + 2H2O
USES:
1. Used in the preparation of amines.
2. Used in making synthetic resins and plastics.
3. It can also be used in making fertilizer.
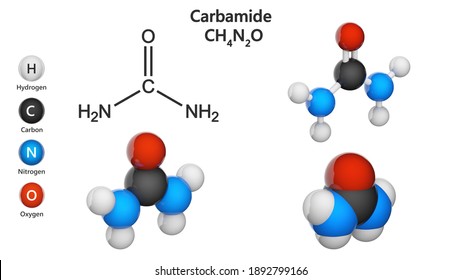
CARBAMIDE/UREA
This is an amide of hydrogen trioxo carbonate (IV) acid. It is produced by compressing CO2 and NH3 at high pressure at 2000C.
EVALUATION
1. Write the structural formula of amide.
2. Give one different between amine and amide.
READING ASSIGNMENT
New School Chemistry by O.Y. Ababio pages 520-521
WEEKEND ASSIGNMENT
1.Tertiary amine is represented as follow
A. R2NH2 B. (CH3)2NH C.R2NH3 D. R2N
2.Which of the following has fishy odour
A. alkanoic acid B. alkanol C. amide D. amine
3.Amide can be regarded as derivatives of …………
A. alkanol B. policarboxylic acid C. monocarboxylic acid D. carbonxamide group
4.During the hydrolysis of amides, one of the following is produced.
A. monocarboxylic acids B. water C. H2SO4 D.NaOH
5. Carbamide is an example of
A. amine B. alkane C. alkyl D. amide
THEORY
1(a). State two (2) physical properties of amide.
(b). How would you prepare ethanamide from an ester.
2(a). State two (2) chemical properties of amide.
(b). How would you identify an example of amine in the laboratory.
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 