Charge flow during electrolysis
The coulomb is the electrolytic unit of charge. A current of one ampere is the rate of flow of charge equal to one coulomb per second.
The charge is calculated from the knowledge of the number of seconds for which a steady current is passed.
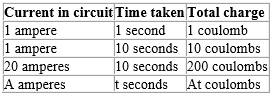
Therefore charge = quantity of electricity (Q) = I × t
Q = I × t
Flow of charge required to liberate 1 mole of element during electrolysis
Electrolysis always produces chemical reactions. Consider a reaction (at cathode) in which one mole of silver Ag+ ions is discharged and deposited.
Ag+ + e– → Ag(s)
In this case, 1 mole of electrons (e–) is required to discharge 1 mole of Ag+ ions to produce 1 mole of silver atoms (Ag(s)). 1 mole of electrons is a large charge and experiments show that it is equal to 96500 coulombs.
Therefore 1 mole of electrons = 96500 coulombs. This is called the faraday.
The number of faradays (moles of electrons) required to liberate 1 mole of an element during electrolysis is deduced from the equation for the electrode reaction.
Examples
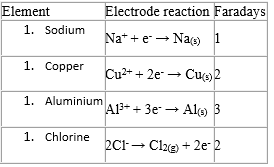
That is, 1 faraday is needed to deposit 1 mole of sodium atoms (23g), 3 faradays to deposit 1 mole of aluminium atoms (27g) and 2 faradays to liberate 1 mole of chlorine gas (71g).
- The mass of an element liberated by 1 coulomb of electricity during electrolysis is called electrochemical equivalent of that element.
- The mass of an element deposited or liberated by 1 faraday during electrolysis is called chemical equivalent of that element.
FARADAY’S LAWS OF ELECTROLYSIS
The laws expressing the quantitative results of electrolysis were first stated by a British chemist called Michael Faraday. The laws assert that the amount (expressed in moles) of an element liberated during electrolysis depends on:
- the time of passing the steady current;
- the magnitude of the steady current passed; and
- the charge on the ion of the element.
The First Law
The fact that the amount of a substance liberated during electrolysis depends upon these factors can be proved by conducting experiments. The product of time (measured in seconds) and the current passed (measured in amperes) gives a measure of electricity known as the quantity of electricity.
Quantity of electricity (Q) = current (I) × time (t)
(coulombs) (amperes) (seconds)
Q = I × t
Because of this relationship, factors (1) and (2) may be included in the same experiment. The experiment to determine the effect of time on the amount of element deposited or liberated is carried out by passing a steady current through a solution of the compound of that element for different lengths of time.
The following table summarises the specimen results obtained by passing a steady current (0.21 amps) through a solution of copper (II) sulphate for 15, 30, 45 and 60 minutes. The last column shows the mass of copper deposited.
Table 61.1 Specimen results for electrolysis of copper (II) sulphate
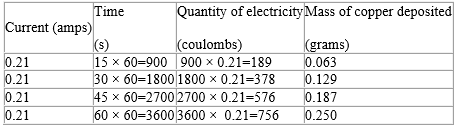
The relationship between the amount of copper deposited and the quantity of electricity passed can be assessed by considering the values in the last two columns in the table. This data may be represented in a graph illustrated in figure 6.4. The shape of the graph shows a straight line passing through the origin. From this fact, it is clear that the mass of copper deposited is directly proportional to the quantity of electricity passed. This is in accordance to what Faraday formulated in his First Law.
Faraday’s First Law of Electrolysis states that the mass of a substance liberated at (or dissolved from) an electrode during electrolysis is directly proportional to the quantity of electricity passing through the electrolyte.
The quantity of electricity is measured in coulombs where a coulomb is the passage of an electric current of one ampere for one second. Let
m be the mass of the substance liberated;
I be the current passed in amperes; and
t be the time in seconds.
We can therefore represent the first law mathematically as:
m α I × t or m = Z × I × t where Z is the proportionality constant referred to as electrochemical equivalent of the substance liberated. Electrochemical equivalent is the mass of a substance (element) liberated by 1 coulomb of electricity during electrolysis.
The Second Law
The third (3) factor mentioned previously as affecting the amount of substance liberated during electrolysis may also be investigated experimentally. Because our interest is the effect of the charge on the ions present in solution, we need to keep the quantity of electricity fixed whilst varying the types of the ions in solution. This may be achieved by passing the same quantity of electricity through two cells, with ions of different charges in each cell.
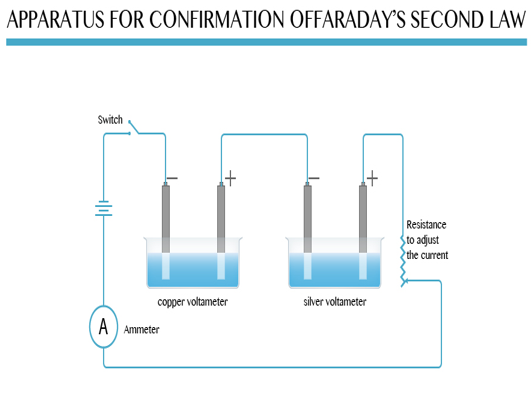
The experiment is conducted using two voltameters. The two voltameters are connected in series as shown. The first one is a copper voltameter and the second is a silver voltameter.
The copper voltameter has copper electrodes in a solution of copper (II) sulphate. Hence, in this voltameter, copper ions are discharged and deposited at the cathode.
The silver voltammeter has silver electrodes in a solution of silver nitrate. The discharged silver ions are deposited at the cathode.
Before the experiment is started, each cathode electrode in the voltameters is cleaned, dried and weighed. The electrodes are then connected to the circuit, after which a suitable current is passed for a measured period of time.
After this, the cathode are removed from the voltameters, cleaned, dried and reweighed. The increase in mass of the two cathode electrodes represents the respective amounts of copper and silver deposited at the cathodes. The quantity of electricity required to deposit one mole of each element is calculated using Faraday’s Second Law of Electrolysis.
Specimen results
| Current flowing | = | 0.45A |
| Duration of current flow | = | 25 minutes |
| Mass of copper deposited | = | 0.221g |
| Mass of silver deposited | = | 0.755g |
The results show that the masses of silver and copper deposited are different. A comparison of the amounts of each of the elements deposited can be made simple by calculating the number of moles of atoms of each of the element deposited.
Thus:Amount of copper deposited = mole = 0.0035 mole
Amount of silver deposited = mole = 0.0070 mole
It is seen that twice as many atoms of silver are deposited as atoms of copper. The difference in amount of each element deposited arises from the difference in charges on ions of the element concerned.
The change on the copper ion (Cu2+) is twice that on the silver ion (Ag+) and therefore twice the quantity of electricity will be required to liberate one mole of copper as for the liberation of one mole of silver.
This relationship is in accordance to Faraday’s Second Law of Electrolysis, which describes the relationship between the amount of element deposited and the charge on the ions of that element.
Faraday’s Second Law of Electrolysis states that when the same quantity of electricity is passed through solutions of different electrolytes the relative numbers of moles of the elements deposited are inversely proportional to the charges on the ions of each of the elements respectively.
In order to discharge one mole of monovalent ions such as H+, Na+, Ag+ and Cl–, 96500 C of electricity are required. This quantity of electricity has been experimentally determined and is known as the Faraday constant.
It represents one mole of electrons, which is the same as the quantity of electrons required to discharge one mole of Ag+ ions to give one mole of silver atom. The validity of Faraday’s Second Law of Electrolysis is evident from the following observations:
- One faraday (IF) discharges one mole of H+, Na+, Ag+, Cl– and OH– ions.
- Two Faradays (2F) discharges one mole of Cu2+, Pb2+, Mg2+, Ca2+, Fe2+, etc ions.
- Three Faradays (3F) discharge one mole of Al3+, Fe3+, etc. ions.
Summarizing the two Laws of Electrolysis
-Chemical equivalent of element =
-Electrochemical equivalent of an element, Z =
Therefore, electrochemical equivalent, Z =
-Mass of substance liberated (m) = ZQ
m = Z × I × t
Since Z = , then m =
But F = 96500 C
Therefore m =
m =
The relation summarizes the two Laws of Electrolysis.
Calculations in electrolysis
- A steady current of 4 amperes is passed through aqueous copper (II) sulphate solution for 1800 seconds using platinum electrodes. Calculate:
- mass of copper deposited
- mass of oxygen liberated
Given: Atomic weight of copper = 63.5
Atomic weight of oxygen = 16
1 Faraday = 96500 C
Clue: Copper is deposited at the cathode and oxygen is liberated at the anode.
Solution
- Cathode reaction: Cu2+ + 2e– → Cu(s)
In this case, 2 Faradays of electricity are required to deposit one mole of copper atom 63.5g.
This means 2 × 96500 C liberates 63.5g of copper.
- Quantity of electricity passed = I×t = 4×1800C. So, if 2×96500 C liberates 63.5g, then 4×1800C will liberate = 2.4g of copper.
Therefore, mass of copper deposited = 2.4g
- Anode reaction: 4OH–→2H2O(l) + O2(g) + 4e–
Solution
The reaction shows that 4 moles of electrons (=4 Faradays) are lost during the reaction process.
Therefore, 4 × 96500 C are needed to liberate one mole (32g) of oxygen.
I.e. 4 × 96500 32g of oxygen gas
- Quantity of electricity flowing = 4 × 1800 C
So if 4 × 96500 C = 32g, then
4 × 1800 C = = 0.6g
Therefore, mass of oxygen liberated = 0.6g
- A current of 3.2 amperes was passed through fused aluminium oxide for 10 minutes. The volume of oxygen given out at the anode was 112cm3 measured at s.t.p. Calculate:
- the mass of aluminium deposited
- the charge of one mole of electrons (The Faraday)
(O=16, Al = 27, molar volume = 22.4 dm3 at s.t.p.)
Note:
1 mole (32g) of oxygen at s.t.p. occupies 22400cm3
Solution
(i) If 32 of oxygen occupies = 22400 cm3, then the weight of oxygen that would occupy 112cm3 = = 0.16g
Let the weight of oxygen liberated be M1 and that of aluminium be M2 and their chemical equivalents be E1 and E2 respectively.
Then, by applying Faraday’s Second Law of Electrolysis, we have the relation
But chemical equivalent =
Therefore, E1 =
E2 =
M1 = 016 E1 = 8
M2 = ? E2 = 9
Substituting the relation in the formula
, we have
M2 =
= 0.18g
\The mass of aluminium deposited = 0.18g
(ii) In this case, we can either use the mass of aluminium deposited or oxygen liberated to find the charge of one mole of electrons (the Faraday):
– The mass of oxygen liberated = 0.16g
– The mass of aluminium deposited = 0.18g
– The charge flow = amperes × seconds = (3.21 × 10 × 60)C
Alternative 1: using the mass of aluminium deposited.
3 moles of electrons are required to deposit 1 mole (27 g) of aluminium atoms
Al3+ + 3e– → Al
If 3 moles of electrons deposit 27g, then 1 mole would deposit g
But, what is the charge of this 1 mole of electrons?
Solution
If 0.18g of Al are deposited by (3.21 × 10 × 60)C, then 9g would require
Alternative 2: using the mass of oxygen liberated
2 moles of electrons are required to deposit one mole (16g) of oxygen atoms
O2- → 2e- + O
If 2 moles of electrons deposit 16g of oxygen atoms, 1 mole deposits 8g.
Solution
If 0.16g requires (3.21 × 10 × 60)C, then 8g would need
Therefore, you can see that both cases give a similar answer (96300C). However, remember that it is difficult to get the exact Faraday constant 996500C) under ordinary practical conditions.
EVALUATION
1.0.222g of a divalent metal is deposited when a current of 0.45A is passed through a solution of its salt for 25minutes.Calculate the relative atomic mass of the metal.
- Calculate the mass of aluminium deposited when a current of 3.0 amperes is passed through an aluminium electrolyte for 2 hours.(Al=27,1 Faraday=96500c).
Read our disclaimer.
AD: Take Free online baptism course: Preachi.com 